Kundu M, Lindsten T, Yang CY, et al. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood. 2008. [Epub ahead of print]
Many dramatic changes of terminal mammalian erythroid cell differentiation occur during reticulocyte maturation, including completion of hemoglobin synthesis, degradation of internal organelles, conversion from aerobic to anaerobic metabolism, and acquisition of a uniform biconcave discoid shape. Recent publications have shed light on the role of autophagy, an intracellular process by which organelles are degraded, in mitochondrial loss during reticulocyte maturation. Two of the studies have demonstrated that knockout mice deficient in Nix, a BH3 domain-only member of the Bcl2 family of proteins, have retarded degradation and clearance of mitochondria in reticulocytes. This inability to degrade mitochondria leads to a shortened erythrocyte survival and anemia (i.e., partially compensated hemolytic anemia). Because Bcl2 and its family of proteins are key regulators of the mitochondrial pathway of apoptosis, the role of Nix in reticulocyte mitochondrial degradation suggests that it can mediate either apoptosis or survival, depending upon circumstances of the individual cell.
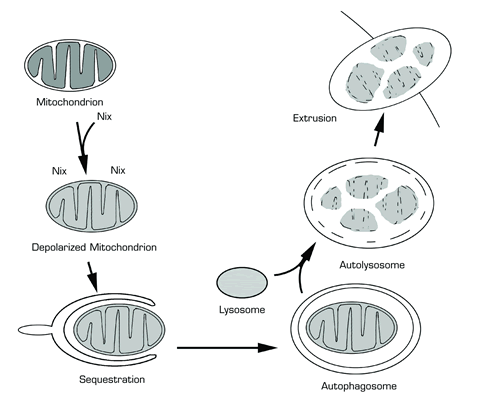
Model of Nix-Mediated Autophagic Clearance of Mitochondria From Reticulocytes. Nix interacts with mitochondria in reticulocytes that have completed heme synthesis. Nix induces depolarization of the inner mitochondrial membrane, and the depolarized mitochondrion is sequestrated in a double membrane structure termed the autophagosome. The autophagosome subsequently fuses with a lysosome forming the autolysosome. Proteolytic enzymes from the lysosome degrade the inner membrane of the autolysosome and partially degrade the sequestered depolarized mitochondrion. The non-degraded contents of the autolysosome are extruded from the reticulocyte.
Model of Nix-Mediated Autophagic Clearance of Mitochondria From Reticulocytes. Nix interacts with mitochondria in reticulocytes that have completed heme synthesis. Nix induces depolarization of the inner mitochondrial membrane, and the depolarized mitochondrion is sequestrated in a double membrane structure termed the autophagosome. The autophagosome subsequently fuses with a lysosome forming the autolysosome. Proteolytic enzymes from the lysosome degrade the inner membrane of the autolysosome and partially degrade the sequestered depolarized mitochondrion. The non-degraded contents of the autolysosome are extruded from the reticulocyte.
In nutrient-deprived cells, autophagy may be an alternative to apoptosis in that essential metabolites required for survival of the nutrient deprivation are salvaged by degrading organelles such as the mitochondria and recycling the crucial metabolic products.1 Similar to nutrient deprivation, maturating reticulocytes reach a crucial stage when death can result if the autophagic process is disrupted. Mitochondria appear to be both degraded and extruded from the maturing reticulocyte by autophagy.2 (See figure.) The failure of mitochondria to undergo autophagy in reticulocytes appears to be detrimental because a similar hemolytic anemia with mitochondria-retaining erythrocytes as found in Nix knockout mice was found in knockout mice with deficiency of Ulk1, the mammalian homologue of atg 1p, a mitochondrial autophagy regulatory protein in yeast.
In Brief
In the sequence of events in mitochondrial autophagy in reticulocytes, Nix acts at the stage of mitochondrial depolarization and targeting for inclusion in autophagosomes. Nix interacts with the outer mitochondrial membrane, leading to loss of inner membrane polarization.3 Reticulocytes from Nix-deficient mice retain polarized mitochondria, but they are localized to areas adjacent to autophagosomes in reticulocytes. This result suggests that targeting of the mitochondria to the autophagosomes may be intact, but Nix’s induction of mitochondrial depolarization is required for normal mitochondrial incorporation into autophagosomes. Further evidence for this role of Nix in mitochondrial depolarization is that chemical depolarization of mitochondria in reticulocytes from Nix-deficient mice leads to their autophagic clearance. Unlike reticulocytes and erythrocytes from Nix-deficient mice, those from Ulk1-deficient mice have retention of ribosomes and mitochondria. Furthermore, the retained mitochondria in the Ulk1-deficient mice are not localized to areas adjacent to autophagosomes, suggesting that Ulk1-deficient mice have a defect at a different point than do Nix-deficient mice in the reticulocyte autophagy pathway.
Further studies of maturing reticulocytes have the potential to determine the specific range, targeting, and fate of the organelles that are removed by autophagy. This information will not only interest those studying erythropoiesis, but also interest those researchers and physicians interested in other cellular processes that involve autophagy, such as differentiation, aging, and survival following chemical or physical stress.