Mutations in tyrosine kinases are a common theme in myeloid leukemia. The hallmark example is the inappropriate activation of Abl through the Bcr-Abl translocation in CML. Mutations in the FLT3 tyrosine kinase are quite common in AML. Recently, point mutations resulting in a valine to phenylalanine substitution at amino acid 617 of the JAK2 kinase (JAK2-V617F) have been found in >90 percent of cases of polycythemia vera (PV), and approximately 50 percent of primary myeloid fibrosis (PMF) and essential thrombocytosis (ET). How can one mutation be associated with three different diseases?
A recent study by Tiedt, et al. paints a fascinating picture of how the mutant gene level can actually influence the malignant phenotype. The authors used elegant genetic engineering to create three mouse models: one with a balanced expression of the wild-type JAK2 and mutant JAK2-V617F, one with relatively high JAK2-V617F, and one with very high JAK2-V617F. The mice developed a hematologic disease influenced by the relative amount of wild-type to mutant allele. Thus, mice expressing balanced expression of wild-type and mutant Jak2 developed an ET-like disease, with increases predominately in platelet counts, splenomegaly, and fibrosis in the bone marrow. Mice that expressed higher levels of mutant JAK2-V617F showed increasing levels of erythroid expansion, with a phenotype that appeared PV-like. A study of 82 patients with myeloproliferative disease and 11 healthy people showed a similar pattern as the mouse model. Quantitative RT-PCR showed the highest mutant: wild-type ratio in cases with PV, followed by PMF, then ET. Expression of the mutant and wild-type JAK2 correlated with the gene copy numbers found in the samples. Thus, cases with PV tended to have samples where the chromosomal number of mutant JAK2 was greater than wild-type.
A variation of this theme has been found in AML cases with the FLT3 mutation. Approximately 15 percent to 30 percent of AML cases with normal cytogenetics harbor FLT3 mutations characterized by a head-to-tail duplication in gene coding for the juxtamembrane region of the protein. The occurrence of this FLT3 internal tandem duplication (FLT3-ITD) alone has had a variable prognostic import across different studies and treatments. However, several studies have now shown that the allelic ratio (the ratio of mutant FLT3-ITD to wild-type allele) drives prognosis.1-3 Cases with predominately mutant FLT3-ITD have a very poor prognosis; cases with predominately wild-type allele tend not to have a poor prognosis.
In Brief
These findings run counter to the conventional (and, perhaps, wrong) wisdom of leukemia being a single clonal event. If AML really is only derived from a single clone, there could only be three possible allelic ratios in respect to the FLT3 mutation in an AML sample: all wild-type; heterogyzgous wild-type and mutant; or all mutant. The fact that one can have a variety of allelic ratios in AML cases suggests that there must be multiple clones in most leukemic cases, each clone having a different state of the three conditions outlined above.
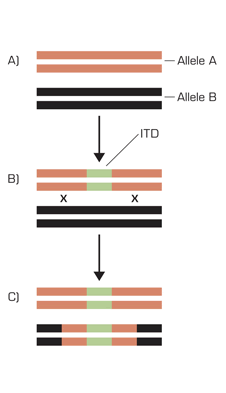
An Example of DNA Repair Causing a Duplication of a Mutated Gene. A: The normal state with two wild-type alleles. B: The acquisition of a mutated sequence (green) leaves the gene in a heterozygous state. C: In the process of repair, a copy of the mutated gene is created and spliced into the other chromosome, leaving two copies of the mutated gene sequence.
An Example of DNA Repair Causing a Duplication of a Mutated Gene. A: The normal state with two wild-type alleles. B: The acquisition of a mutated sequence (green) leaves the gene in a heterozygous state. C: In the process of repair, a copy of the mutated gene is created and spliced into the other chromosome, leaving two copies of the mutated gene sequence.
Of interest, the allelic data suggest not only the case of a loss of the normal FLT3 (resulting in one mutant gene, no wild-type), but in some cases, a duplication of the mutant gene. How does a patient develop two copies of a mutant gene? Bad luck twice? In some cases of malignancy, wild-type alleles are dropped through chromosome loss (for example, deletion of an arm of chromosome 17 eliminates a copy of the p53 tumor suppressor gene). This loss of heterozygosity, however, does not appear to be the case in the JAK2 and FLT3 story. Here, rather, the process of chromosomal repair causes a duplication of the mutant allele (see Figure). In this situation, a double-stranded DNA break takes place, and, in order to facilitate repair, a second copy of one of the genes is made. In the process of repair, one of the copies of the genes is lost. If the recombination process selects the wild-type gene to duplicate, then the cell has two copies of the wild-type gene, and order is restored. If, in this process, the wild-type gene is selected, then the cell has two copies of the mutated gene.
Thus, the mere presence of a mutated tyrosine kinase does not tell the whole story in myeloid malignancies. The dosage level of the mutated gene can affect the disease phenotype and the biology of response. Once again in science and medicine, the more we know, the more we need to know.
In Brief
Of interest, the allelic data suggest not only the case of a loss of the normal FLT3 (resulting in one mutant gene, no wild-type), but in some cases, a duplication of the mutant gene. How does a patient develop two copies of a mutant gene? Bad luck twice? In some cases of malignancy, wild-type alleles are dropped through chromosome loss (for example, deletion of an arm of chromosome 17 eliminates a copy of the p53 tumor suppressor gene). This loss of heterozygosity, however, does not appear to be the case in the JAK2 and FLT3 story. Here, rather, the process of chromosomal repair causes a duplication of the mutant allele (see Figure). In this situation, a double-stranded DNA break takes place, and, in order to facilitate repair, a second copy of one of the genes is made. In the process of repair, one of the copies of the genes is lost. If the recombination process selects the wild-type gene to duplicate, then the cell has two copies of the wild-type gene, and order is restored. If, in this process, the wild-type gene is selected, then the cell has two copies of the mutated gene.
Thus, the mere presence of a mutated tyrosine kinase does not tell the whole story in myeloid malignancies. The dosage level of the mutated gene can affect the disease phenotype and the biology of response. Once again in science and medicine, the more we know, the more we need to know.
References
Competing Interests
Dr. Radich indicated no relevant conflicts of interest.