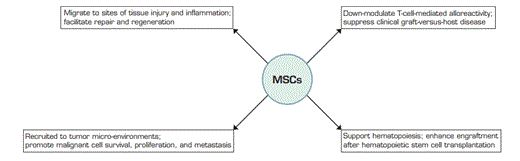
Mesenchymal stem (or stromal) cells (MSCs) are culture-derived, non-hematopoietic, adherent progenitors that are defined by specific immunophenotypic features and the ability to differentiate into adipocytes, chondrocytes, or osteoblasts. In vivo, MSCs can migrate to sites of tissue injury and inflammation where they produce trophic and growth factors that facilitate repair and regeneration. MSCs also support hematopoiesis; they are relatively non-immunogenic and can down-modulate T-cell-mediated alloreactivity.1 Pilot and phase II studies in allogeneic hematopoietic stem cell transplantation (HSCT) suggest that donor or mismatched, “third party,” marrow-derived MSCs are safe and can enhance engraftment in certain patients or treat corticosteroid-refractory graft-versus-host disease (GVHD). Importantly, MSCs are also recruited to tumor microenvironments, and studies in murine or human xenograft tumor models show that systemic delivery or co-implantation of MSCs can promote malignant cell survival, proliferation, and/or metastasis.2-4 Thus, the safety of MSCs in patients undergoing HSCT for malignancies remains a major concern.
The report by LeBlanc, et al. describes a multicenter, non-randomized phase II trial of donor or third-party marrow MSCs for severe, steroid-refractory acute GVHD after myeloablative or non-myeloablative HSCT for a hematologic malignancy (78 percent), solid tumor (4 percent), or non-malignant disease (12 percent). One intravenous infusion of MSCs induced a complete response (CR) in 27 of 55 patients (49 percent), and CR occurred in 30 patients overall (55 percent). Compared with patients without CR, those with CR had significantly lower one-year transplant-related mortality (37 percent vs. 72 percent) and higher two-year survival (53 percent vs. 16 percent). Response was not related to GVHD grade, MSC source, or total MSC dose. No acute or late side effects were reported; relapse occurred in three of 43 patients (7 percent) with hematologic malignancy. In the randomized controlled trial by Ning, et al., HLA-matched donor MSCs were co-transplanted with marrow and/or peripheral blood stem cells on day zero after myeloablative conditioning for hematologic malignancies. Patients were randomized by age, disease type, stage, and prognosis. Only 10 of 15 patients allocated to the treatment arm received MSCs; their engraftment was not enhanced, but only one developed GVHD, compared with eight of 15 non-MSC control patients. The study was closed early because six of the 10 patients who received MSCs relapsed (including five recurrences by day 150), compared with three relapses in the 15 non-MSC patients. The three-year disease-free survival rates for the MSC and non-MSC groups were 30 percent and 66.7 percent, respectively (log rank p=0.035).
Culture-Derived MSCs, Which are Defined by Specific Immunophenotypic Features and Their Ability to Differentiate into Adipocytes, Chondrocytes, or Osteoblasts in vitro, Exhibit Pleiotropic Functions in vivo.
Culture-Derived MSCs, Which are Defined by Specific Immunophenotypic Features and Their Ability to Differentiate into Adipocytes, Chondrocytes, or Osteoblasts in vitro, Exhibit Pleiotropic Functions in vivo.
In Brief
These observations add to the growing experience of using culture-expanded MSCs in HSCT. The results of LeBlanc, et al. are highly encouraging. If confirmed in current randomized clinical trials, MSCs could offer the safest and most effective salvage therapy option for steroid-resistant acute GVHD. This enthusiasm must be tempered, however, by the observations of Ning, et al. that remind us that MSCs can promote malignant cell survival and growth. Reassuringly, high relapse rates were not observed in a similar, but non-randomized, study of 46 patients with hematologic malignancies undergoing myeloablative HSCT with MSC co-transplantation on day zero,5 nor have increased relapse rates been reported after administering MSCs for GVHD. Moreover, the results of Ning, et al. may not be broadly applicable because their study groups were small and the technical limitations that prevented optimal donor MSC expansion could have introduced confounding variables. Additional clinical and pathobiological studies are needed to address whether MSCs enhance disease recurrence after HSCT, especially when co-transplanted on day zero, and if this might occur through direct cell-cell interactions, paracrine effects, and/or suppression of graft-versus-tumor alloreactivity.
References
Competing Interests
Dr. Linenberger indicated no relevant conflicts of interest.