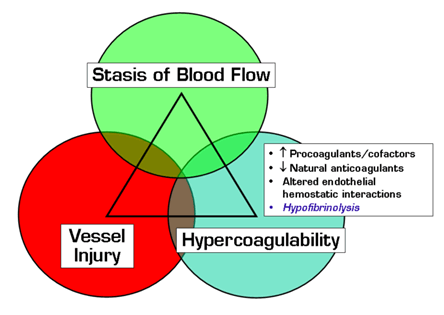
Acquired and familial hypercoagulable states include a number of well-characterized disorders that affect procoagulant proteins or cofactors, natural anticoagulants, and other mediators of hemostatic interactions with vascular endothelium. Although affected individuals are inherently prothrombotic, symptomatic deep vein thrombosis (DVT), pulmonary embolism (PE), and other types of venous thromboembolism (VTE) often occur only in the setting of additional "provocative" risk factors such as pregnancy, surgery, immobilization, obesity, advanced age, and/or exogenous female sex hormones. Since unprovoked VTE occurs in healthy young people with no identifiable predisposing hypercoagulable condition, other mechanisms must exist, and many studies have searched for prothrombotic defects related to impaired fibrinolysis. Aside from rare familial thrombotic dysfibrinogenemias and plasminogen deficiencies and a mild association with elevated levels of thrombin-activatable fibrinolysis inhibitor, no clear causal linkage has yet been established between VTE risk and specific abnormalities of tissue plasminogen activator (tPA), plasminogen activator inhibitor type 1, or α2-antiplasmin.
To further address this question, Meltzer, et al. performed a case-control study to determine whether hypofibrinolysis, as measured by a plasma clot lysis time (CLT) assay, might be an independent or cooperative risk factor for VTE. This investigation follows preliminary observations that patients in the Leiden Thrombophilia Study (LETS) with a plasma CLT at or above the 90th percentile had a two-fold increased risk of DVT.1 The CLT assay, performed by adding exogenous tissue factor and tPA to citrated plasma and measuring the time from half-maximal clot formation to half-maximal clot dissolution, reflects global plasma fibrinolytic potential and the interplay between the coagulation and fibrinolytic systems.1 In the current study, CLTs on 2,090 patients with a first idiopathic or provoked DVT and/or PE enrolled in the Multiple Environmental and Genetic Assessment (MEGA) were compared with 2,564 controls. Plasma samples were taken three months after discontinuation of anticoagulant therapy or one year after the VTE event. Hypofibrinolysis, defined as a CLT within the highest quartile of control values (i.e., the longest CLTs), was associated with an odds ratio for VTE (adjusted for age and sex; ORadj) of 2.4 in the absence of other prothrombotic risks. When hypofibrinolysis was combined with oral contraceptive pill (OCP) usage, immobilization, or factor V Leiden heterozygosity, a synergistic effect on VTE risk was observed with ORadj of 21.8, 10.3, and 8.1 compared to ORadj without hypofibrinolysis of 2.6, 4.3, and 3.5, respectively. In the control group, longer CLTs were seen with increasing age, male sex, increasing body mass index, diabetes, and the prothrombin 20210A mutation.
In Brief
This large population-based study observed a 2.4-fold relative risk of a first VTE among individuals with hypofibrinolysis, which, by definition, was present in 25 percent of healthy controls. This is equivalent to the individual risks associated with exogenous female hormones, hyperhomocystinemia, and heterozygous prothrombin 20210A mutation. Concurrent immobilization, OCP usage, or factor V Leiden heterozygosity significantly augmented the VTE risk with hypofibrinolysis, reminiscent of the synergizing effects of additional clinical and genetic risks with other hypercoagulable states and consistent with the concept that VTE often results from overlapping predisposing conditions (see Figure). Additional data suggest that hypofibrinolysis may play a role in the pathogenesis of VTE with increasing age, obesity, diabetes, and the prothrombin 20210A mutation. Further studies are required, however, to define the genetic and functional mechanisms responsible for the impaired fibrinolytic potential as determined by the CLT assay. Prospective validation studies are also warranted to confirm that a prior VTE event does not affect CLT results.
From a clinical perspective, important next questions include:
Is hypofibrinolysis associated with the risk of recurrent VTE, and can it be used to guide duration of anticoagulation?
Is hypofibrinolysis related to VTE risks with other prothrombotic conditions (e.g., cancer, myeloproliferative disorders, antiphospholipid syndrome), and might it provide new perspectives on prevention and treatment in these settings?
Can the CLT assay be validated for widespread clinical applicability, and what clinical and pharmacologic variables might confound its interpretation?
References
Competing Interests
Dr. Linenberger indicated no relevant conflicts of interest.