Halcyon was the mythical bird that was said to breed in a nest floating at sea at the winter solstice, charming the wind and waves into calm. As an adjective, halcyon denotes a period of time in the past that was idyllically happy and peaceful. The halcyon days of erythropoietin supplementation lasted for about 15 years beginning in June 1989 when the U.S. Food and Drug Administration approved the recombinant human protein for treatment of anemia of chronic renal failure. The beginning of the end of those tranquil times occurred about five years ago with publication of a randomized study suggesting an adverse outcome for patients with head and neck cancer undergoing radiation therapy who received recombinant human erythropoietin for treatment of anemia.1 A subsequent study in mainly non-anemic patients with breast cancer receiving first-line chemotherapy also demonstrated inferior progression-free survival for those receiving the recombinant hormone.2 And although subsequent meta-analysis has suggested an overall survival benefit for cancer patients receiving supplemental erythropoietin,3 the calm could not be restored, and our once naïve view of erythropoietin as the simple, innocent regulator of erythropoiesis has been replaced by the current world-weary (sinister) concept of erythropoietin as a pleiotropic cytokine that effects a variety of physiologic and pathophysiologic processes. In addition to its primary function of regulating erythropoiesis, erythropoietin stimulates angiogenesis (both physiological and pathophysiological), upregulates tissue renin, enhances production of endothelin, and stimulates endothelial and vascular smooth muscle cell proliferation. Now compelling data from Tong and colleagues suggest a role for endogenous erythropoietin in neovascular complications of diabetes.
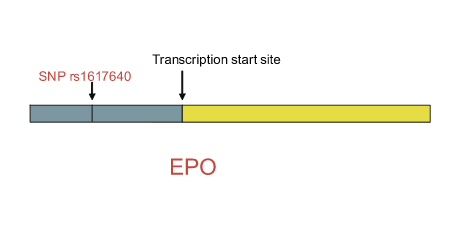
A remarkably high concordance rate (80 percent to 90 percent) exists between proliferative diabetic retinopathy (PDR) and end-stage renal disease (ESRD), and these complications show strong familial aggregation. Together, these observations suggest a genetic influence (susceptibility or resistance). Linkage studies have identified several potential loci, including a modifier of nephropathy on chromosome 7q21. To avoid the potentially confounding effects of phenotypic heterogeneity (especially as it applies to diabetic nephropathy), Tang, et al. used a case-controlled design that focused on patients with both PDR and ESRD. Given the important role of neovascularization in the pathophysiology of both PDR and ESRD, the investigators hypothesized that genes involved in angiogenesis effected susceptibility to these complications of diabetes. To test this hypothesis, 19 single nucleotide polymorphisms (SNPs) from 11 candidate genes were analyzed for allelic association. The initial genotyping involved 374 patients with both PDR and ESRD and 239 age- and ethnicity-matched controls from a Utah European-American type 2 diabetes cohort. Except for SNP rs1617640, located on 7q21, 1,125 bp upstream of the erythropoietin gene transcription start site (see Figure), no association was found. The linkage of SNP rs1617640 with diabetic microvascular complications was supported by analysis of two other large, independent type 1 diabetes cohorts. Compared to the GG genotype, erythropoietin concentration in the vitreous body was 7.5 times higher in samples from individuals with the TT risk genotype, and the T allele enhanced expression of a luciferase reporter construct by 25-fold compared to the G allele. The TT genotype appears to modulate erythropoietin expression by creating a binding site for transcription factors EVI1/MEL1 or AP1. Analysis of gene expression in murine models provided additional support for a role for erythropoietin in both diabetic retinopathy and nephropathy.
Relationship Between SNP rs1617640 and the Erythropoietin Gene. The SNP is located 1,125 bp upstream of the EPO transcription site. The findings of Tong, et al. suggest an additive allele-dose model with the heterozygous risk allele being GT and the homozygous risk allele being TT. The T allele appears to enhance EPO expression by creating a matrix match with EVI1/MEL1 or AP1 transcription enhancer binding sites.To simplify the Figure, the region of EPO containing the coding sequence is illustrated by the uninterrupted yellow rectangle and is not drawn to scale. The blue rectangle represents noncoding sequence upstream of the EPO start site and is not drawn to scale.
Relationship Between SNP rs1617640 and the Erythropoietin Gene. The SNP is located 1,125 bp upstream of the EPO transcription site. The findings of Tong, et al. suggest an additive allele-dose model with the heterozygous risk allele being GT and the homozygous risk allele being TT. The T allele appears to enhance EPO expression by creating a matrix match with EVI1/MEL1 or AP1 transcription enhancer binding sites.To simplify the Figure, the region of EPO containing the coding sequence is illustrated by the uninterrupted yellow rectangle and is not drawn to scale. The blue rectangle represents noncoding sequence upstream of the EPO start site and is not drawn to scale.
Recombinant erythropoietin is used extensively to treat patients with the anemia of renal failure (many of whom have diabetic nephropathy), and patients whose hemoglobin concentration is maintained above 13.5 gm/dl have a higher rate of cardiovascular complications than those maintained at a hemoglobin concentration below 11.5 gm/dl.4 The studies of Tong and colleagues provide a plausible explanation for the observed concordance and genetic predisposition to PDR and ESRD and send yet another cautionary note to physicians who prescribe the recombinant protein.
References
Competing Interests
Dr. Parker indicated no relevant conflicts of interest.