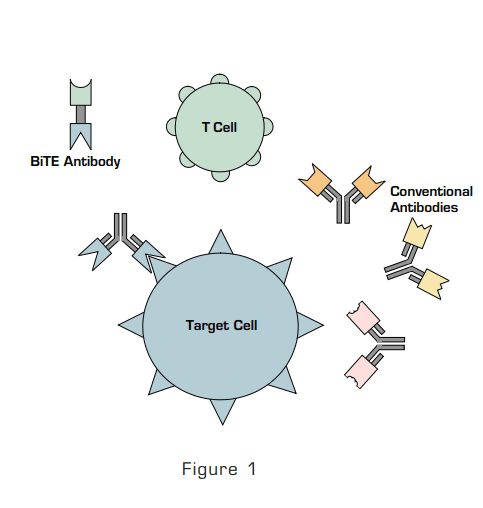
Figure 1: The Fab of Conventional Antibodies Bind to Their Target Cells but do not Bind to T Cells as T Cells Lack a Receptor for the Fc Portion of the Antibody. BiTE antibody-like molecule is a single-chain polypeptide consisting of a binding site for the target antigen and a CD3 binding site that binds to CD3.
Figure 1: The Fab of Conventional Antibodies Bind to Their Target Cells but do not Bind to T Cells as T Cells Lack a Receptor for the Fc Portion of the Antibody. BiTE antibody-like molecule is a single-chain polypeptide consisting of a binding site for the target antigen and a CD3 binding site that binds to CD3.
It has been decades since immunotherapy of tumors was proposed, but the initial excitement was rapidly dampened by failure to find tumor-specific antigens as these potential targets were shared with normal tissues. Furthermore, administration of antibodies against these antigens has been frequently followed by antigen modulation resulting in rapid refractoriness of the tumor and the need for a relatively large concentration of antibodies. While cell-mediated immunotherapy based on in vitro expansion of antigen-trained T cells and dendritic cells have been more promising for EBV-initiated tumors such as certain lymphomas and Hodgkin lymphoma, this laborious and expensive approach has been limited to a few specialized centers, and clinical studies of other therapies designed to elicit a T-cell response, such as vaccination and anti-CTCL4 therapy, have thus far been disappointing.
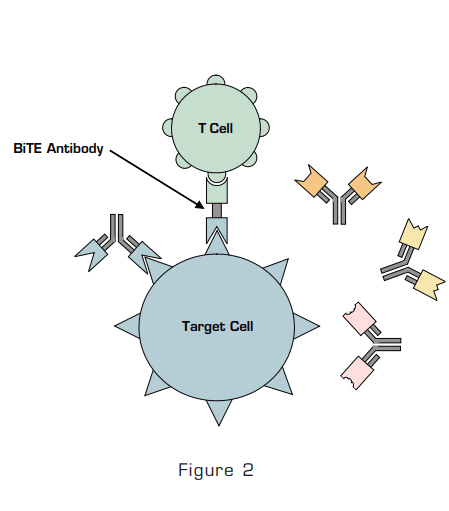
Figure 2: BiTE Antibody Binds to the Target Cell and to the CD3 Antigen on T Cells.
Figure 2: BiTE Antibody Binds to the Target Cell and to the CD3 Antigen on T Cells.
T cells lack an Fcγ receptor and thus cannot be recruited or activated by conventional antibodies. Bispecific T-cell Engagers (BiTE) are a novel construct of single-chain bispecific antibody-like molecules with one end having specificity for a tumor-expressing antigen and the other for a T-cell antigen. As shown in the Figure, a BiTE antibody tethers T cells to a target cell determined by its tumor-expressing antigen specificity, and this direct engagement elicits cytotoxicity. Blinatumomab is a BiTE antibody that has dual specificity for the T-cell CD3 antigen and the universal B-cell antigen CD19. Bargou and coworkers from Germany describe the first human trial of a BiTE antibody. The authors treated 38 patients with various B-cell non-Hodgkin lymphomas at increasing dose levels of blinatumomab in this phase I/II study. The majority of patients had follicular lymphoma (41 percent) or mantle cell lymphoma (38 percent). All had received prior therapy, with a median of three prior therapies (range 1 to 12). Eighty-seven percent had received rituximab. Blinatumomab was administered as a continuous intravenous infusion over four to eight weeks at dose levels of 0.0005 to 0.06 mg/m2. There were four complete and seven partial responses, all of which occurred at doses of 0.015 mg/m2 or higher. All seven patients treated at the highest dose level (0.06 mg/m2) responded. The majority of tumor regression occurred during the first four weeks of therapy. The longest duration of CR was 13+ months in a patient with mantle cell lymphoma and three additional patients had responses lasting longer than six months. The most common toxicities were fever, chills, lympho- and leukopenia, and increased C reactive protein levels. These side effects were most common in the first week of treatment. Eight patients discontinued therapy due to adverse events. Five of the eight had central nervous system events, including confusion, cerebellar symptoms, and seizures, but all symptoms were reversed with discontinuation of therapy. CD19 positive cells rapidly vanished from the peripheral blood and remained undetectable during therapy. Peripheral T-cell numbers also initially vanished but then returned to baseline and in some patients were increased.
In Brief
This report by Bargou, et al. may radically change the applicability and efficacy of cell-mediated immunotherapy of cancer. The BiTE antibody approach bypasses many of the mechanisms cancer cells use to evade cytotoxic T cells. Binding to CD3 activates cytotoxic T cells and effectively recruits additional T cells to tumor tissue. In addition, the cell kill is limited to the cells with the target antigen. Thus, low levels of BiTE antibody should be effective, and indeed the amount of blinatumomab associated with response in this trial was an amazing 5-log-fold lower than the amount of rituximab required for response. Additional work in a larger number of patients will be required to ascertain the response rate and duration of response. In addition, potential toxicities of this therapy will need to be better defined; for example, the drastic decline of B cells would be expected to lead to the usual complications of immunosuppression. A more convenient schedule of administration would also be desirable. However, this is indeed a very promising new therapy, and BiTE antibodies to other antigens, including CD33 and CEA, are already in the process of development.
Competing Interests
Drs. Gregg and Prchal indicated no relevant conflicts of interest.