Actuarial Probability of Relapse After Bone Marrow Transplantation for Early Leukemia According to Type of Graft and Development of GVHD.
Actuarial Probability of Relapse After Bone Marrow Transplantation for Early Leukemia According to Type of Graft and Development of GVHD.
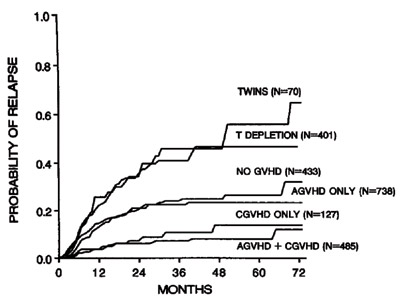
Investigators from the IBMTR have now revisited this clinical concept to ask whether patients benefit from an unrelated donor (URD) transplant because of a stronger GVL effect. The authors analyzed 4,099 patients with AML, ALL, and CML undergoing a myeloablative allogeneic HCT from a URD (8/8 HLA-matched, n=941) or HLA-identical sibling donor (n=3158). In multivariate analysis, URD transplant recipients with AML had a higher risk for transplant-related mortality and relapse than those receiving transplants from HLA-identical sibling donors. This difference was not seen in patients with ALL or CML. Chronic GVHD was associated with a lower relapse risk in all diagnoses. Leukemia-free survival (LFS) was decreased in patients with AML without acute GVHD receiving URD transplant, but was comparable to those receiving HLA-identical sibling transplants in patients with ALL and CML. In patients without GVHD, multivariate analysis showed similar risk of relapse, but decreased LFS for URD transplants for all three diagnoses. The authors concluded that the risk of relapse was the same (ALL, CML) or worse (AML) in URD transplant recipients compared to HLA-identical sibling transplant recipients, suggesting a similar GVL effect.
The GVL effect has not been evaluated as frequently in URD transplants as in sibling transplants. Today, approximately one-third of patients in need of HCT have an available HLA-identical sibling to serve as a donor. The growth of donor registries worldwide has improved the overall chance that a patient who lacks a family donor will have a suitable URD. In a previous Diffusion selection (The HematologistMarch/April 2008 issueMarch/April 2008 issue), I discussed two major studies relevant to this subject. One by Lee and coworkers clearly highlighted the fact that there is a 10 percent loss of survival with any mismatch (although of marginal significance for HLA-DQ), but that classical risk factors including patient age, race, disease tage, and CMV status were as predictive of survival as donor HLA matching.2 The other by Shaw and coworkers confirmed in a large number of patients that DPB1 functions as a classical transplantation antigen. The increased risk of GVHD associated with HLA-DPB1 mismatching was accompanied by a lower risk of relapse.3
In Brief
Thus the study by Ringden and coworkers basically confirms the strong anti-leukemic effect of GVHD. However, even if transplant with URD is associated with more GVHD, this does not seem to translate to better leukemic control. The caveat of this study is the fact that authors included only 8/8-matched URD (HLA-A, -B, -C, -DRB1 matched). Thus, they exclude DPB1, which is the allele reported by Shaw and coworkers to be linked to lower relapse and GVL effect.
References
Competing Interests
Dr. Socié indicated no relevant conflicts of interest.