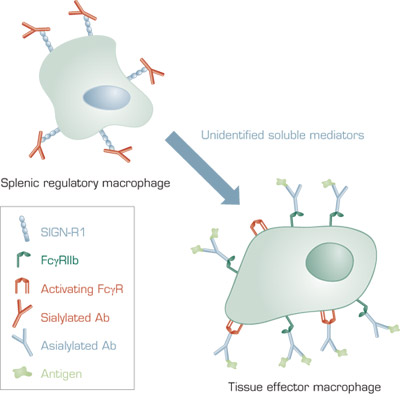
A Model for the Anti-Inflammatory Activity of 2,6-Sialylated Fc. In mice, 2,6-sialylated Fc present in IVIG binds to SIGN-R1 on splenic macrophages. This results in secretion of unidentified soluble anti-inflammatory mediators, which bind to effector macrophages at tissue sites of inflammation. The effector macrophages increase surface expression of the inhibitory FcγRIIB receptor. FcγRIIB competes for binding of antibody–antigen complexes to activating FcγR and increases the threshold concentration of antibody–antigen complexes required for inflammation. In humans, the receptor for 2,6-sialylated Fc is DC-SIGN and the regulatory cells are dendritic cells that are not restricted to the spleen.
A Model for the Anti-Inflammatory Activity of 2,6-Sialylated Fc. In mice, 2,6-sialylated Fc present in IVIG binds to SIGN-R1 on splenic macrophages. This results in secretion of unidentified soluble anti-inflammatory mediators, which bind to effector macrophages at tissue sites of inflammation. The effector macrophages increase surface expression of the inhibitory FcγRIIB receptor. FcγRIIB competes for binding of antibody–antigen complexes to activating FcγR and increases the threshold concentration of antibody–antigen complexes required for inflammation. In humans, the receptor for 2,6-sialylated Fc is DC-SIGN and the regulatory cells are dendritic cells that are not restricted to the spleen.
The mechanism underlying the paradoxical anti-inflammatory effect of IVIG has been the subject of considerable investigation. The Y-shaped immunoglobulin G (IgG) molecule consists of two antigen-binding arms and a stem that contains the Fc domain. Fc domains mediate binding of Igs to cellular receptors, which links antigen recognition to cellular responses. Both the antigen-binding regions and the Fc domain have been implicated in the anti-inflammatory action of IVIG. Ligation of activating Fc receptors for IgG (FcγRs) mediates pro-inflammatory responses, such as phagocytosis and tumor-cell killing as well as auto-inflammatory responses in systemic lupus erythematosus, rheumatoid arthritis, and other disorders. However, inhibitory FcγRs, including FcγRIIb in mice, have been identified that mediate anti-inflammatory responses.
Previously, Ravetch and co-workers identified Fc fragments that demonstrated efficacy in several murine inflammatory disease models. Subsequently, they identified anti-inflammatory activity in a small subpopulation of IVIG. This subpopulation consisted of IgG with N-linked glycan at Asn297 that contained a terminal sialic acid in α2,6 linkage to the penultimate galactose.3 In the present study, the authors explored the mechanism of the anti-inflammatory activity of 2,6-sialylated Fc. To do so, they used an inflammatory disease model in which mice were injected with sera from K/BxN mice, which spontaneously develop antibody-mediated arthritis and soft-tissue inflammation. K/BxN sera produces quantifiable paw swelling that can be used to measure the therapeutic effect of IVIG. Initial experiments indicated that splenic macrophages were necessary for the efficacy of IVIG in this model. Then the authors searched for a receptor for 2,6-sialylated Fc among candidate macrophage cell surface carbohydrate-binding proteins. The central observation of the paper was that antibodies to the lectin SIGN-R1 inhibited the anti-inflammatory properties of IVIG. Furthermore, IVIG was ineffective in mice lacking SIGN-R1. Additionally, sialylated Fc bound poorly to a macrophage cell line lacking SIGN-R1 compared to a cell line expressing SIGN-R1. Adoptive transfer of splenocytes from normal (C57BL/6), IVIG-treated mice to SIGN-R1 knockout mice protected against K/BxN sera challenge. However, adoptive transfer was ineffective in mice lacking FcγRIIb. This result indicates that macrophages containing FcγRIIb are the effector cells in the anti-inflammatory response mediated by IVIG.
Humans do not have SIGN-R1, but express a related molecule, DC-SIGN, which contains a carbohydrate recognition domain that is homologous to SIGN-R1. In contrast to SIGN-R1, which is expressed on macrophages, DC-SIGN is expressed in dendritic cells. Heterologous expression of SIGN-R1 or DC-SIGN in Chinese hamster ovary cell lines resulted in saturable binding of sialylated Fc. This binding was inhibited by mannan, which is consistent with the proposal that Fc binding by SIGN-R1 and DC-SIGN includes recognition of the penultimate mannose in the sialylated glycan.
In Brief
The results of this study provide new insight into the anti-inflammatory properties of IVIG and identify a novel pathway that potentially could be targeted in the treatment of autoimmune diseases.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.