The advance of therapy for pediatric ALL is a success story that oncology can revel in; it also places the bar very high for the rest of the oncology landscape. Whereas pediatric ALL therapy can cure upward of 80 percent of patients, success in adult ALL is stuck at a rate of ~40 percent. Indeed, some pediatric trials are using risk stratification to give less therapy to some cases. This is a mind-boggling luxury for those physicians who work in the adult arena.
Two recent papers have direct relevance to how we should be treating adult ALL. The first, by Fielding, et al., examines the fate of adult ALL cases that relapse after therapy. The paper is a clear, illuminating, and utterly depressing read. The overall five-year survival for cases who relapsed was only 7 percent. This bleak result was not influenced by the type of therapy the patients initially received (i.e., chemotherapy or transplantation). For those who received chemotherapy following transplant, only 4 percent survived. Patients who were "salvaged" with allogeneic transplant fared slightly better, with a survival of 23 percent. The paper’s overwhelming conclusion is that there is one good chance to cure patients, and that more toxicity up front might be a reasonable tradeoff for a decreased relapse rate.
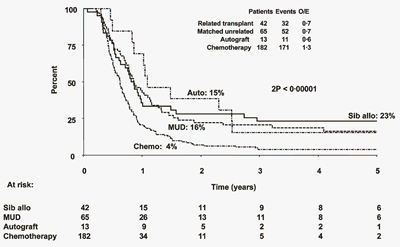
The recent results of the MRC/ECOG trial of front-line therapy for ALL suggest that allogeneic transplantation might offer the best chance for cure. In this trial, patients who had a donor were eligible for transplant in first CR; those without a donor were potentially randomized to autologous or chemotherapy. Of the 1,929 patients entering the trial, 1,031 were HLA typed, and 443 patients had an HLA-matched related donor, while 588 did not. Analysis was performed based on the donor status. The study suggests that, for Ph-chromosome-negative patients, more intense therapy is associated with greater success, with overall survival in allogeneic >autologous> chemotherapy (~53 percent vs. 45 percent vs. 37 percent, respectively). The advantage of allogeneic transplantation did not appear to be as strong in high-risk ALL, due to an unusual amount of non-relapse mortality among the transplant recipients. There are, of course, the usual caveats attached to such complex studies: worries about dropout rate, differences in the percentages of patients acceptable to randomization, analysis by "intent" rather than actual treatment, etc.
Overall Survival of Ph-Negative Adult ALL Cases Divided by the Presence or Absence of a Donor. Figure from Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in ALL patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993) . Blood. 2008;111:1827-33.
Overall Survival of Ph-Negative Adult ALL Cases Divided by the Presence or Absence of a Donor. Figure from Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in ALL patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993) . Blood. 2008;111:1827-33.
Overall Survival of Ph-Negative Adult ALL Cases Divided by the Presence or Absence of a Donor. Figure from Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in ALL patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood. 2007. [Epub ahead of print]
Overall Survival of Ph-Negative Adult ALL Cases Divided by the Presence or Absence of a Donor. Figure from Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in ALL patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood. 2007. [Epub ahead of print]
In Brief
A reasonable interpretation of the data would suggest:
Adult ALL patients should be offered a clinical trial whenever possible. The prevailing therapy is not good enough to be a "standard" therapy.
Allogeneic transplantation should be considered in patients who are at good risk. Given that, at many centers, unrelated transplant results are similar to related, both avenues of donors should be considered.
Physicians should employ the most rigorous methods of minimal residual disease detection, either flow cytometric or molecular (i.e., immunoglobulin heavy-chain VDJ rearrangements). Physicians should demand that their centers adopt technology that can detect at least one leukemic cell in a background of >1,000 normal cells, since this level of detection has been shown to reliably predict relapse.
More money needs to be spent studying the biology of ALL. Except for Ph+ ALL, the "targeted" therapeutic revolution has passed over ALL. Much discovery is needed in adult ALL if we wish to ever approach the success of our pediatric colleagues in this disease.
Competing Interests
Dr. Radich indicated no relevant conflicts of interest.

![Overall Survival of Ph-Negative Adult ALL Cases Divided by the Presence or Absence of a Donor. Figure from Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia (ALL) the greatest benefit is achieved from a matched sibling allogeneic transplant in first complete remission (CR) and an autologous transplant is less effective than conventional consolidation/maintenance chemotherapy in ALL patients: final results of the international ALL trial (MRC UKALL XII/ECOG E2993). Blood. 2007. [Epub ahead of print]](https://ash.silverchair-cdn.com/ash/content_public/journal/thehematologist/6/4/10.1182_hem.v6.4.6138/2/m_6138b.gif?Expires=1769083935&Signature=dCHSq5WpHyUkqonVaOIffAxt-mth~DQqSqaTnzypxgNieAQDIWRW7aDJ4A5WL7uX70T5JqN80EKTpR5TtAzZ6G8~i50aL9UtiA1EldXAeuXV7OX61r~JPzGcvdEK1pEJoVnfWWTPXJNj~cfikvku3-TU-UL8M4yH8IHgpRFEVKJzPMETb1Dx2dp2MojugbJNT7B1SG4MsxCapbyvuY-BJh4wDoUExB6MyksqJPf5c-QbMR2FDKKjakwt3Ns5f0BCQv~hDaMPUkBaiaP8ytr~wjYBovw940KGkbig32sdyOK8Xb1q9dVseidd9Jr4c5OQYhM8JSUhxL-Z9Vx9CnPsIw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)