Three leukocyte adhesion deficiency (LAD) disorders have been described. The most common, type I, is associated with recurrent infections and is due to absent β2 integrin (CD18) on leukocytes. Subjects present with delayed umbilical cord separation, frequent bacterial infections, and marked leukocytosis. The least common, LAD-II, is characterized by deficient or absent protein fucosylation and mild neutrophil dysfunction, but severe physical and mental developmental problems. LAD-III is associated with recurrent infections and mucosal bleeding.
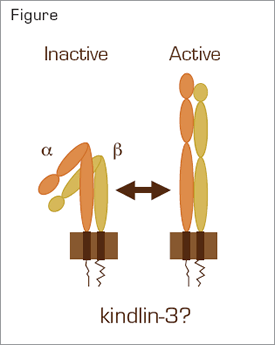
Integrin Activation. Intracellular signaling converts an integrin aß heterodimer from an inactive, bent conformation to an active conformation that can bind ligands. Several molecules, including Rap1 and talin, have been implicated in the formation of a complex that binds to NXXY/F motifs within the cytoplasmic tail of the ß subunit during inside-out signaling. The role of kindlin-3 in integrin activation is unknown. However, it has also recently been shown to bind to NXXY/F motifs.3
Integrin Activation. Intracellular signaling converts an integrin aß heterodimer from an inactive, bent conformation to an active conformation that can bind ligands. Several molecules, including Rap1 and talin, have been implicated in the formation of a complex that binds to NXXY/F motifs within the cytoplasmic tail of the ß subunit during inside-out signaling. The role of kindlin-3 in integrin activation is unknown. However, it has also recently been shown to bind to NXXY/F motifs.3
Malinin et al., from Tatiana Byzova’s laboratory at The Cleveland Clinic, studied two siblings with recurrent infections, leukocytosis, mucosal bleeding, hepatosplenomegaly, and osteopetrosis. Although consistent in some respects with LAD-III, both subjects had additional abnormalities that have not been described in this disorder, including profound defects in platelet aggregation in response to thrombin, ADP, and phorbol 12-myristate 13-acetate. Additionally, there were marked deficiencies in platelet adhesion to fibrinogen and expression of activated β3 integrin αIIbβ3. However, expression of resting αIIbβ3 was normal. These results are consistent with a defect in inside-out signaling of αIIbβ3. Expression of β2 integrins on the subjects’ neutrophils was normal, excluding the diagnosis of LAD-I. However, neutrophil function, including adhesion, was abnormal in several assays. Additionally, defects in adhesion and integrin activation were present in the lymphocytes of the subjects. Consistent with the presence of osteopetrosis, bone marrow-derived mesenchymal stem cells from the subjects produced abnormally high amounts of bone and cartilage in vitro.
The authors considered defects in several candidate genes implicated in integrin activation as the cause of the disorder. Using western blots, bioassays, or gene sequence analyses, they found no abnormalities of talin, filamin, vinculin, components of the protein kinase C pathway, L-selectin shedding, extracellular signal regulated kinase, p38, RAP1, RAPL, or CALDEG-GEF1. However, acting on the recent discovery that disruption of the kindlin3 gene in mice is associated with platelet dysfunction and defective integrin activation,1 the authors found a nonsense mutation in exon 16 of the KINDLIN 3 gene of both subjects. Importantly, Epstein-Barr virus-immortalized lymphocytes from both subjects, which were defective in adhesion assays, were normalized by transfection with a kindlin-3 encoding cDNA. Both subjects underwent allogeneic bone marrow transplantation, which resolved all of the clinical and in vitro abnormalities.
The authors propose calling the disorder in this family integrin activation deficiency disease (IADD) because it appears phenotypically distinct from LAD-III. Additionally, LAD-III has been associated with either RAP1 or CALDEG-GEF1 deficiency, which was not identified in the present study. However, kindlin-3-deficiency has been described in three patients carrying a diagnosis of LAD-III who also had osteopetrosis,2 making the distinction between LAD-III and IADD unclear at the present time.
In Brief
This study by Malinin et al. is important because it provides further evidence that kindlin-3 is an important component of the mechanism of integrin activation. How kindlin-3 functions in this process is poorly understood and the work described in this paper surely will stimulate additional research in this area.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.