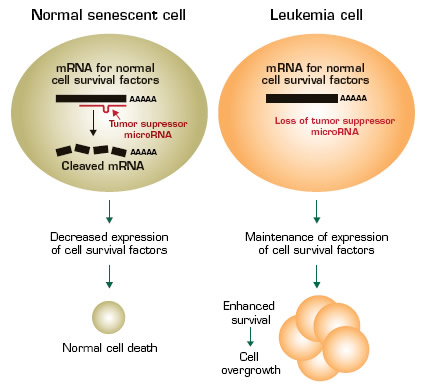
MicroRNAs (miRs) are small, 18-to-24 nucleotide noncoding RNAs that regulate stability and/or translation into protein of partially complementary target messenger RNA (mRNA) molecules. The manner in which miRs regulate translation is complex. MiRs are thought to cause degradation of their target mRNAs, but other mechanisms may also exist and there are even rare reports of miRs enhancing translation. For any given miR, there may be multiple potential target mRNAs, and for any given mRNA, there can be multiple miRs that affect its translation. Like mRNAs encoding for proteins, miRs have well-defined developmental and cell-type-specific expression patterns. Also, like other genes, genes encoding miRs play a role in tumor suppression and are mutated in malignancies.1
This recent publication from Carlo Croce’s laboratory1 demonstrates that miR-29b is mutated in leukemia and that restoration of miR-29b expression prevents the ability of leukemia cells from growing in an immunodeficient mouse host. The miR-29 family consists of four members: miR-29a, mir-29b-I, miR-29b-II, and miR-29c. Each of these has the same short “seed” sequence and, thus, is predicted to target the same genes. Down-regulated expression of different miR-29 family members has been reported in multiple malignancies including lung cancer, breast cancer, chronic lymphocytic leukemia (CLL), and in AML with chromosome translocations involving fusion of the MLL1gene, in particular with the t(6;11) and t(9;11) translocations.
The investigators searched for potential miR-29 target genes that were down-regulated with enforced overexpression of miR-29b, and identified multiple genes that inhibit cell death and/or promote cell survival and proliferation including MCL-1 (bcl2-related myeloid cell leukemia sequence 1), CXXC6 (also named TET oncogene 1), and CDK6 (cyclin dependent kinase 6). CXXC6 is a translocation partner of MLL in some forms of AML. Thus, miR-29b may act in normal cells to prevent cancer, and lack of this miR in leukemia leads to over-expression of genes that promote cancer. Analysis of 45 primary AML samples revealed an inverse correlation between the expression levels of MCL-1 and miR-29b. The locus encoding miR-29b-1/ miR-29a is on chromosome 7q32 in a region that is frequently deleted in myelodysplastic syndromes (MDS) as well as secondary AML, and miR-29a and -29b levels are lower in AML with monosomy 7 compared to other karyotypes.
In order to test whether overexpression of miR-29b could be used to block cancer progression, the investigators used a xenogeneic model system in which cells from a human leukemia cell line were transplanted into immunodeficient mice. Within two weeks, these mice had measurable tumors. In contrast, in mice that received injections of synthetic miR-29b, as opposed to controls that received unrelated miRs, the average tumor weights were significantly lower and two tumors disappeared completely. It is not yet known which target genes decreased tumor growth, and since the investigators used an immortalized cell line for this work, it is not clear how these findings specifically relate to primary AML. However, in a separate, related study from the same laboratory,2 enforced overexpression of miR-29b in primary AML blasts led to decreased expression of multiple DNA methyltransferase genes, which caused global DNA hypomethylation resulting in increased expression of tumor suppressors.
In Brief
Therefore, whether miR-29b inhibits leukemia cell growth by direct effects on potential target genes or by global demethylation and activation of tumor suppressors, these data suggest that loss of miR-29b promotes leukemia, and synthetic miR-29b may be a novel therapeutic agent in AML. Such an approach would not necessarily be used as a single agent because cancer cells have evolved ways of escaping control by microRNAs.
References
Competing Interests
Dr. Krause indicated no relevant conflicts of interest.