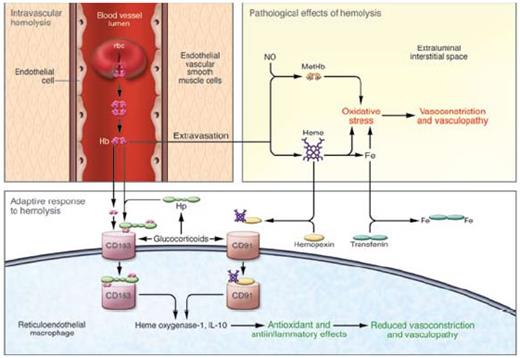
A Model of Intravascular Hemolysis: Pathological and Adaptive Responses. Free hemoglobin can lead to pathological vasoconstriction and vasculopathy by limiting NO bioavailability and potentiating oxidative stress. An adaptive response occurs when hemoglobin is bound to haptoglobin and cleared by CD163 or heme binds to hemopexin cleared by CD91with induction of cytoprotectants including heme oxygenase-1. In turn, an anti-inflammatory/anti-oxidant response protects the vasculature.2 Copyright 2009 by American Society for Clinical Investigation. Reproduced with permission of American Society for Clinical Investigation.
A Model of Intravascular Hemolysis: Pathological and Adaptive Responses. Free hemoglobin can lead to pathological vasoconstriction and vasculopathy by limiting NO bioavailability and potentiating oxidative stress. An adaptive response occurs when hemoglobin is bound to haptoglobin and cleared by CD163 or heme binds to hemopexin cleared by CD91with induction of cytoprotectants including heme oxygenase-1. In turn, an anti-inflammatory/anti-oxidant response protects the vasculature.2 Copyright 2009 by American Society for Clinical Investigation. Reproduced with permission of American Society for Clinical Investigation.
In Brief
These studies aid in understanding toxicities due to free plasma hemoglobin and support potential therapeutic applications for haptoglobin in preventing them. As nicely pointed out by Kato2 in an accompanying commentary to this article (Figure), corticosteroids may improve hemolysis by enhancing clearance of hemoglobin through induction of haptoglobin and CD163. Furthermore, in sickle cell disease, haptoglobin levels are low. Would increased levels modulate sickle vasculopathy? In turn, if hemoglobin/haptoglobin complexes induce heme oxygenase-1, another adaptive protective stratagem would be triggered in hemolytic disease.3 Patients with intravascular hemolysis and vascular damage in the brain and kidney due to thrombotic thrombocytopenic purpura benefit from plasma exchange not only by removing ADAMTS13 antibodies and replacing ADAMTS enzyme, but also by adding fresh haptoglobin, preventing hemoglobin and heme catalyzed oxidative stress. Possibly, studies on haptoglobin polymorphisms in vascular diseases may reflect these underlying pathophysiologies. For now, there are few pharmacologic mechanisms to increase plasma haptoglobin, but future research should focus on this important mop for hemoglobin.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.