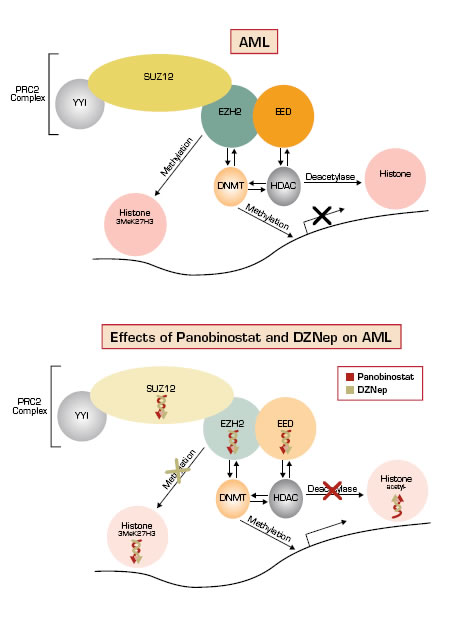
Epigenomic modifications play important roles in cancer initiation and progression. Major mechanisms include aberrant promoter hypermethylation of CpG DNA and histone modifications, such as deacetylation and methylation, which lead to silencing of tumor suppressor genes. These changes can collaborate to exhibit mutual effects on chromatin structure and gene expression.1 Agents that target the deregulated epigenome, including DNA methyltransferase (DNMT) inhibitors and histone deacetylase (HDAC) inhibitors, have demonstrated efficacy against a subset of hematologic malignancies, and they are now being combined in clinical trials.2 However, recent clinical correlative studies in patients with myelodysplastic syndrome and acute myeloid leukemia (AML) have raised questions about whether cytotoxicity induced by DNMT and HDAC inhibitors are directly attributable to de-repression of epigenetic silencing or related to other mechanisms.3 EZH2 is part of the polycomb repressive complex 2 (PRC2) and is the major enzyme that catalyzes H3 lysine 27 trimethylation (3MeK27H3) resulting in gene silencing.4 Antagonists of EZH2 provide an additional route for epigenetic modification therapy, especially because 3MeK27H3 markings can silence genes without promoter hypermethylation.1 To date, no EZH2 antagonists have been tested in the clinic.
Fiskus et al., working in the laboratory of Kapil Bhalla at the Medical College of Georgia Cancer Center, investigated the effects of 3-deazaneplanocin A (DZNep, an EZH2 antagonist) and panobinostat (an HDAC inhibitor) in preclinical models of AML. In AML cell lines, they observed that single agent DZNep induced cell cycle arrest and apoptosis, reduced clonogenic survival, and specifically decreased levels of 3MeK27H3 (no effect on 3MeK9H3 or 3MeK79H3). In AML mice models, they observed significantly increased survival with both agents compared to single-agent therapy. In primary AML cells, they observed that exposure to both agents decreased protein levels of EZH2, SUZ12, and EED, which are all core components of PRC2, decreased levels of 3MeK27H3 (Figure), and were more lethal than when exposed to a single agent. Both agents were observed to induce more differentiation than single agents in AML cell lines. Importantly, they observed relatively little effect on apoptosis, EZH2, SUZ12, and EED protein levels, and 3MeK27H3 levels in normal CD34+ bone marrow progenitor cells treated with both agents.
In Brief
It is intriguing to consider that the synergy of DZNep and panobinostat are primarily related to their mutual effects on EZH2 and modulation of leukemia-promoting genes. However, DZNep is an S-adenosylhomocysteine hydrolase inhibitor and may, therefore, affect a variety of processes that require methyl transfer. In addition, observations in other models suggest that EZH2 regulates a wide variety of genes, including those involved in immunoresponse and autocrine inflammation.5 Thus, the precise mechanisms of EZH2 depletion and in vivo cytotoxicity of DZNep remain to be fully elucidated. Nevertheless, these observations support the rationale for studying EZH2 antagonist and HDAC inhibitor combination therapy in patients with AML. It will be important that these trials include collaborative, high-throughput genome-wide assays that measure high-resolution DNA methylation and histone modifications in order to correlate clinical responses with changes in candidate epigenetic marks and to identify alternative mechanisms related to off-target effects.
References
Competing Interests
Drs. Miller and Linenberger indicated no relevant conflicts of interest.