Diamond-Blackfan anemia (DBA) is a rare inherited hypoplastic anemia presenting in infancy. DBA shares many phenotypic characteristics with the acquired 5q- syndrome subtype of myelodysplastic syndrome (del [5q] MDS): macrocytic anemia leading to red blood cell transfusion dependence, reticulocytopenia, relative preservation of the neutrophil and platelet counts, and a risk of transformation to acute myeloid leukemia. In 1999, DBA was the first congenital marrow failure syndrome in which ribosomal protein (RP) deficiency was implicated as a cause.1 Approximately 25 percent of DBA patients carry a mutation in RP S19, and mutations in several additional ribosomal proteins are now linked to the disease. Active research questions in DBA include why red blood cells are particularly susceptible to the perturbations of protein synthesis and whether there are defects in the extra-ribosomal function(s) of the mutated RPs that contribute to the disease phenotype.
Aside from their clinical and laboratory similarities, the first biologic convergence between DBA and del (5q) MDS arose from elegant studies by Benjamin Ebert et al. first reported at the ASH Plenary Scientific Session in 2007. Using an RNA-interference approach, they identified haploinsufficiency of RPS14, whose gene is located in the commonly deleted region (CDR) on the long arm of chromosome 5 as a critical gene responsible for the hematopoietic (especially erythroid) defect observed in del (5q) MDS.2
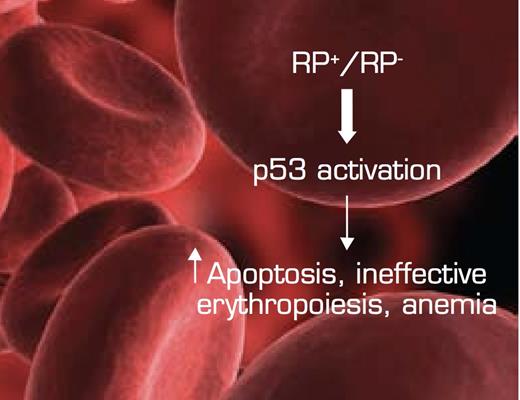
The Convergence of Bone Marrow Failure Syndromes Such as del (5q) MDS and DBA, Ribosomal Protein (RP) Haploinsufficiency, and Induction of p53. In this model, reduced RP gene dosage triggers stabilization/activation of the p53 pathway in the erythroid department, resulting in increased apoptosis and ineffective erythropoiesis/anemia. Figure adapted from McGowan KA, Li JZ, Park CY, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008; 40:963-70. (Figure 7)
The Convergence of Bone Marrow Failure Syndromes Such as del (5q) MDS and DBA, Ribosomal Protein (RP) Haploinsufficiency, and Induction of p53. In this model, reduced RP gene dosage triggers stabilization/activation of the p53 pathway in the erythroid department, resulting in increased apoptosis and ineffective erythropoiesis/anemia. Figure adapted from McGowan KA, Li JZ, Park CY, et al. Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet. 2008; 40:963-70. (Figure 7)
In their seminal report, Barlow and colleagues from Cambridge have generated a murine model of del (5q) MDS exploiting novel chromosomal engineering approaches using Cre-loxP recombination to delete the syntenic regions located on mouse chromosomes 11 and 18 equivalent to the CDR on human chromosome 5.3 Although a series of deletions on mouse chromosome 11 did not produce appreciable changes in hematopoiesis, deletion of the gene interval Cd74-Ni67 on mouse chromosome 18 recapitulated several clinicopathologic features of del (5q) MDS: macrocytic anemia, prominent dyserythropoiesis, and monolobated megakaryocytes. Mice with deleted Cd74-Ni67 also demonstrated marked decreases in erythroid and myeloid progenitors, increased bone marrow cell apoptosis, thrombocytopenia, and reduced marrow cellularity. Although the latter two features may not represent the most common presentation of del (5q) MDS, this murine model of large segmental chromosomal deletion is a robust approximation of several features of the disease. It is noteworthy that RP S14 is among the eight genes contained in the Cd74-Ni67 interval, and it therefore remains a strong candidate gene for the del (5q) syndrome.
When tumor suppressor p53 (trp53) deficiency was introduced by intercrossing Cd74-Ni67-deleted mice with trp53-/- mice, several key hematopoietic defects were corrected, including the deficit in eythroid and myeloid progenitor populations, thrombocytopenia, bone marrow dysplasia, and macrocytosis. The reliance on activation of the p53 pathway for the del (5q) phenotype dovetails nicely with data from different venues indicating that ribosome “stress” leads to induction of p53: in a zebrafish model with deficiency of RPS19, defective erythropoiesis involves activation of p53,3 and in recently published murine models of mutated RP S6, RP S19, and RP S20, a dark-skin phenotype as well as a reduced erythrocyte count were dependent on stabilization/activation of p53.4
In Brief
These data indicate a central role for p53 in the molecular pathobiology of del (5q) MDS. Hemizygosity for genes encoding ribosomal proteins that result in disruption of ribosome biogenesis and activation of p53 is now emerging as a shared paradigm among bone marrow failure syndromes (Figure). It will be of interest to determine whether lenalidomide acts in del (5q) MDS through targeting of the p53 pathway and whether other therapeutics can be used to exploit biologic checkpoints related to this tumor suppressor.
References
Competing Interests
Dr. Gotlib is the principal investigator on a pilot study of lenalidomide in Diamond-Blackfan anemia and receives research funding from Celgene, Inc.