There was a time when we thought we understood disease-damaged tissue. Take for example diabetic retinopathy, one of the most important causes of visual loss worldwide. In the case of non-proliferative diabetic retinopathy, we were taught that chronic hyperglycemia led to vascular changes resulting in retinal injury, ischemia, and acellular retinal capillaries. While this description of pathogenesis is correct, the underlying pathophysiology may be many miles of capillaries away. Recent interest in endothelial progenitor cells (EPCs) has led to their documented importance in the maintenance of homeostasis and repair of the vasculature, and abnormal or reduced numbers have been associated with many diseases. Furthermore, experiments with parabiotic mice have demonstrated that normal mice contribute to healing of wounds in diabetic mice, and young mice contribute to healing in older mice.
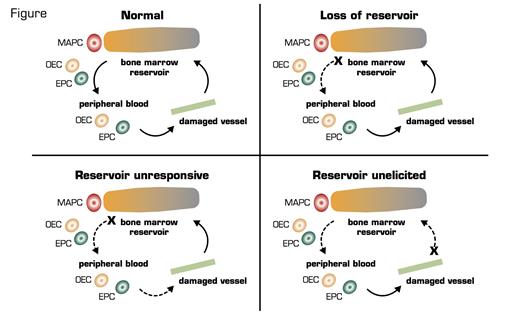
EPCs are bone marrow-derived progenitors and can home to areas of damage for the reparative process. Busik et al. used a rat model of type II diabetes and demonstrated that diabetic individuals have fewer EPCs in the circulation, and their EPCs have less migratory potential thus resulting in less optimal healing. So far, not much surprise. However, they were able to demonstrate that the reason for fewer EPCs is due to bone marrow neuropathy, and, importantly, the neuropathic changes in the bone marrow occurred before the diabetic retinopathy. In rats with diabetes for four months, there was a dramatic reduction of nerve terminals in the marrow. Using a transplantation model with green fluorescent protein-labeled cells, this denervation was associated with increased numbers of EPCs in the marrow but decreased numbers in circulation. Moreover, these diabetic animals’ marrow had fewer EPC colonies, and these cells had lower proliferation. These data suggest that the neuropathy led to hyporesponsiveness of the marrow in releasing EPCs to vascular-damaged signals (Figure). Importantly, the authors were able to demonstrate that this denervation also led to loss of the normal circadian rhythm that releases EPCs. This reduction of peak EPC release from the intrinsic clock also leads to a decrease in the healing process.
Possible Mechanisms of Lack of Tissue Repair From EPCs From the Marrow. (EPCs: endothelial progenitor cells; MAPCs: multipotent adult progenitor cells; OECs: late outgrowth endothelial cells.) Figure courtesy of Robert Storms.
Possible Mechanisms of Lack of Tissue Repair From EPCs From the Marrow. (EPCs: endothelial progenitor cells; MAPCs: multipotent adult progenitor cells; OECs: late outgrowth endothelial cells.) Figure courtesy of Robert Storms.
In Brief
This interesting article ties in the importance of the bone marrow as a reservoir for tissue repair. Mobilization of marrow EPCs occurs following activation of peripheral noradrenergic neurons and release of norepinephrine, which suppresses osteoblast activity. These data suggest that the marrow is not responsive. However, other scenarios could be that the reservoir is not elicited. That is, normal damage signals are not sent to the marrow, or perhaps there is a loss of reservoir as with aging. Of course, none of these scenarios are exclusive of the other. These data do lead to a new potential therapeutic target for non-proliferative diabetic vascular complications. Finally, while this paper is very interesting, it does beg the question of what led to the marrow denervation in the first place. More questions to be answered!
Competing Interests
Dr. Chao indicated no relevant conflicts of interest.