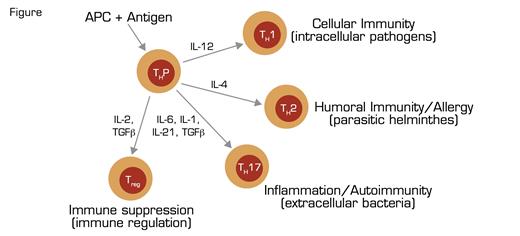
T-Cell Differentiation Naïve, Precusor T Cells (THP) Recognize Antigens in the Context of HLA Proteins Expressed by Antigen Presenting Cells (APC). Subsequent expression of cytokines orchestrates differentiation into particular effector subclasses (T helper 1, TH1; T helper 2, TH2; T helper 17, TH17; regulatory T, Treg). Each effector subclass is involved in a response to a particular infectious/immune process (shown in parentheses), and each has a cytokine signature (e.g., interferon γ in the case of TH1, IL-4 in the case of TH2, IL-17 in the case of TH17, and TGFβ in the case of Treg) that mediates activity. Tregs control the immune response by repressing the activity of the effectors through several mechanisms including secretion of suppressor molecules and expression of cytokine and chemokine receptors that rob effector cells of essential activation cues. The same transcription factors that integrate the environmental cues for the effector cells are involved in orchestrating the Treg response, thereby ensuring class-specific control of immune-mediated inflammation.
T-Cell Differentiation Naïve, Precusor T Cells (THP) Recognize Antigens in the Context of HLA Proteins Expressed by Antigen Presenting Cells (APC). Subsequent expression of cytokines orchestrates differentiation into particular effector subclasses (T helper 1, TH1; T helper 2, TH2; T helper 17, TH17; regulatory T, Treg). Each effector subclass is involved in a response to a particular infectious/immune process (shown in parentheses), and each has a cytokine signature (e.g., interferon γ in the case of TH1, IL-4 in the case of TH2, IL-17 in the case of TH17, and TGFβ in the case of Treg) that mediates activity. Tregs control the immune response by repressing the activity of the effectors through several mechanisms including secretion of suppressor molecules and expression of cytokine and chemokine receptors that rob effector cells of essential activation cues. The same transcription factors that integrate the environmental cues for the effector cells are involved in orchestrating the Treg response, thereby ensuring class-specific control of immune-mediated inflammation.
The critical importance of CD4+ CD25+ Foxp3+ regulatory T cells (Tregs) in immune homeostasis and protection against autoimmunity is illustrated by the consequences of inherited mutation of the X-linked gene Foxp3, a member of the forkhead/winged helix family of transcription factors. Foxp3 is the master regulator of both development and function of Tregs, and inherited mutation of the gene underlies the immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome that is characterized clinically by severe autoimmune phenomena including Coombs-positive autoimmune hemolytic anemia, immune thrombocytopenic purpura, autoimmune enteropathy, dermatitis, thyroiditis, and type 1 diabetes. The disease is often fatal within the first two years of life. In patients with IPEX and in mice with mutant foxp3 (called scurfy), there is uncontrolled T-cell proliferation with infiltration of affected tissue by T helper 1 (TH1) and T helper 2 (TH2) lymphocytes and drastically elevated production of TH1 and TH2 cytokines (i.e., interferon-γ and IL-4, respectively) (Figure).
These findings provide compelling indirect evidence that both cellular (TH1) and humoral/allergic (TH2) immune responses are regulated by Tregs (Figure). Whether the other major effectors of the immune response (i.e., TH17 lymphocytes that mediate inflammation/autoimmunity) (Figure) are also under the control of Tregs, however, has been speculative. Now a team of investigators from Memorial Sloan-Kettering Cancer Center and the University of Washington, led by Alexander Rudensky, have demonstrated that like TH1 and TH2 lymphocytes, the activity of TH17 cells is suppressed by Tregs. Moreover, using a cleverly designed conditional knockout model in which expression of the transcription factor Stat3 is inactivated in cells expressing Foxp3, they showed that the same transcription factor, Stat3, that is the key initiator of TH17 differentiation endows Tregs with the capacity to repress TH17 responses. These remarkable findings are in keeping with previous studies indicating that the same transcription factors that are required for Treg-mediated suppression of TH1 and TH2 immune responses are necessary for activation, differentiation, and proliferation of these T-cell subsets. Together, these findings suggest an integrated model in which environmental cues determine a particular immune response by activating the same transcription factor axes in both effector and regulatory arms of the system. This elegant counterbalancing system ensures appropriate class-specific control of infectious processes while concordantly regulating immune-mediated inflammation.
Why should hematologists care about Tregs? In fact, we don’t have to look beyond the most recent meeting of our Society to find an exciting clinical correlate. In a paper presented during the Plenary Scientific Session of the 2009 ASH Annual Meeting, Martelli and colleagues reported that freshly isolated donor Tregs protected against recipient graft-versus-host disease while allowing for robust immune reconstitution in patients undergoing haploidentical hematopoietic stem cell transplantation who received a concurrent inoculum of donor mature T cells.1 Interest in the therapeutic use of Tregs is not limited to hematology, however, as oncologists, immunologists, and endocrinologists have initiated clinical trials that involve manipulation of Tregs to suppress or enhance the immune response. Rigorous, insightful basic research, such as that of Chaudhry et al., allows us to understand more clearly the fundamental workings of Tregs so that we can optimally exploit for clinical benefit the remarkable properties of these master regulators of the immune response.
References
Competing Interests
Dr. Parker indicated no relevant conflicts of interest.