In clinical studies, the investigators showed that synovial fluids from rheumatoid arthritis patients contained 0.2-1.0 micron diameter CD41 (GPIIb) expressing vesicles, while 19/20 osteoarthritic patients had no such particles. Interestingly, no intact platelets were detected in the synovial fluids;
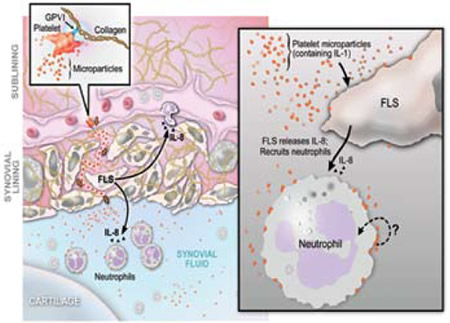
Proposed Pathway for GPVI-Dependent Participation of Platelets in Arthritis via Microparticles. GPVI-expressing platelets activated by collagen produce copious amounts of IL-1-rich microparticles (MPs) (left panel and inset). The precise anatomic location of platelet activation and the route by which microparticles enter the joint (dotted red line in the left side of the figure where the microparticles are going across the synovial lining) remain unknown. Platelet MPs (~0.2-1 μm in diameter), detectable at high levels in inflammatory synovial fluid, interact with tissue cells including fibroblast-like synoviocytes (FLS) and synovial fluid leukocytes (right panel). This interaction elicits further inflammatory effector functions from target cells, thereby amplifying synovitis. In the case of FLS, platelet MPs promote elaboration of IL-8 and other mediators that are capable of leukocyte chemoattraction to the joint (right panel). Platelet microparticles attached to neutrophils, as found in diseased synovial fluid, may also stimulate neutrophil effector functions, although this remains to be established (question mark, right panel). Figure courtesy of S. Moskowitz and D. Lee.
Proposed Pathway for GPVI-Dependent Participation of Platelets in Arthritis via Microparticles. GPVI-expressing platelets activated by collagen produce copious amounts of IL-1-rich microparticles (MPs) (left panel and inset). The precise anatomic location of platelet activation and the route by which microparticles enter the joint (dotted red line in the left side of the figure where the microparticles are going across the synovial lining) remain unknown. Platelet MPs (~0.2-1 μm in diameter), detectable at high levels in inflammatory synovial fluid, interact with tissue cells including fibroblast-like synoviocytes (FLS) and synovial fluid leukocytes (right panel). This interaction elicits further inflammatory effector functions from target cells, thereby amplifying synovitis. In the case of FLS, platelet MPs promote elaboration of IL-8 and other mediators that are capable of leukocyte chemoattraction to the joint (right panel). Platelet microparticles attached to neutrophils, as found in diseased synovial fluid, may also stimulate neutrophil effector functions, although this remains to be established (question mark, right panel). Figure courtesy of S. Moskowitz and D. Lee.
CD45-positive leukocytes seemed to be carrying microparticles into the joint. In a K/BxN serum transfer mouse model of inflammatory arthritis, platelet depletion, but not blockade of the ADP receptor P2YR12 by clopidogrel or absence of thromboxane or GPIIb, cooled the joint flames. Local rather than systemic platelet activation seemed to play a role as fibroblast-like synoviocytes and their extracellular matrix-associated proteins triggered microparticle release. Platelet GPVI and its associated gamma chain of the Fc receptor proved essential in microparticle release responding to collagen and collagenrelated peptides. Mechanistically, platelet microparticles could elicit a range of cytokines including IL-6 and IL-8 from fibroblast-like synoviocytes. The activation of these cells (so abundant in the rheumatoid pannus) by platelet microparticles was related to platelet-associated IL-1 (Figure).
In Brief
This article suggests that inhibiting platelet GPV1 may be useful in treating rheumatoid arthritis. One would think that IL-1 antagonism would be very efficacious, but apparently platelet microparticle-associated IL-1 is difficult to antagonize. As Zimmerman points out in an accompanying article,5 we usually don’t think about platelets playing a significant role in rheumatoid arthritis. Since intact platelets are not found in the rheumatoid arthritis joint space, how do the microparticles get there? Could they be hitchhiking on transmigrating white cells? Boilard et al. propose that platelets release infiltrating microparticles after GPVI activation when they contact collagen. The microparticles enter the joint space via fenestrations in permeable subsynovial capillaries (Figure). This manuscript adds to the evidence for the critical role of platelets in inflammation and spurs me to speculate whether excessive platelet transfusions may be providing kindling for inflammatory fires in our thrombocytopenic patients.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.