For the hematologist lecturing on hemostasis, it has never been a pleasant task to march students through the intrinsic pathway and then tell them that the initiating reactions in the pathway apparently are not necessary in vivo. The intrinsic pathway starts when blood comes into contact with a variety of negatively charged surfaces, including non-physiological materials such as glass, kaolin, and ellagic acid. Binding to these surfaces induces a conformational change in factor XII (FXII) that allows it to proteolytically activate prekallikrein (PK). PK proteolytically activates FXII, producing a positive feedback loop that amplifies the system and leads to activation of FXI and cleavage of high-molecular-weight kininogen (HK) by kallikrein. Cleavage of HK in turn produces the inflammatory mediator bradykinin.
These contact activation reactions represent the “activated” part of the activated partial thromboplastin time. However, severe deficiencies of FXII, PK, or HK do not produce a bleeding diathesis, leading to the teaching that the contact system is somewhat of an orphan in search of a function. In fact, the first patient who was diagnosed with FXII deficiency, John Hageman, died of pulmonary embolism. However, recent work in several laboratories has indicated that the contact system may contribute to the formation of pathologic intravascular thrombi.1
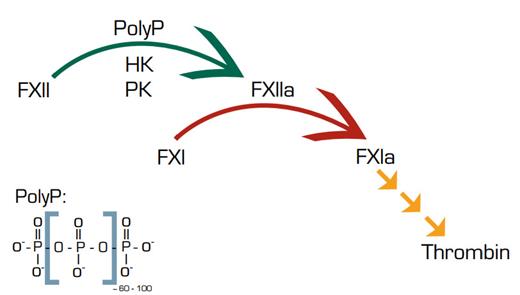
Now Müller et al. in the laboratory of Thomas Renné, in collaboration with the laboratory of James Morrissey, provide evidence that platelet or E. coli-derived polyphosphate (PolyP) provides a surface for activation of FXII and the contact system and promotes a prothrombotic/proinflammatory diathesis in mice. PolyP is linear polymer of ~60 to 100 orthophosphate units that is found widely in prokaryotes and eukaryotes. Recently, PolyP was identified in platelet dense granules2 and found to have procoagulant properties and antifibrinolytic properties.3
Müller et al. found that plateletderived PolyP-dependent FXII activation resulted in proteolysis of HK and release of bradykinin, leading to increased vascular permeability. FXII-deficient and bradykinin receptor-deficient mice were resistant to PolyP-induced vascular permeability. Intravenous infusion of platelet-derived PolyP produced lethal pulmonary embolism in normal mice, which was significantly reduced in FXII-deficient and/or FXI-deficient mice. A specific inhibitor of FXII also protected against PolyP-induced pulmonary embolism. A thrombin receptor agonist, TRAP6, also produced lethal pulmonary embolism in normal mice, which was significantly decreased in FXII-deficient mice. Degradation of PolyP with phosphatases inhibited contact activation in vivo and in vitro and decreased platelet PolyP-induced thrombosis in mice. Additionally, E. coli-derived PolyP initiated the activation of FXII, KK, and FXI and the production of bradykinin. Finally, addition of PolyP restored the plasma clotting defect of Hermansky–Pudlak syndrome platelets, which lack PolyP.
In Brief
This study indicates that PolyP is a potentially important mediator of platelet-driven proinflammatory and procoagulant disorders. PolyP, thus, is a logical target for the development of a new class of antithrombotic agents.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.