"Circumstances do not make the man, they reveal him." – James Allen
Characteristic congenital limb malformations (phocomelia) are synonymous with the medical tragedy wrought by thalidomide in pregnant women in the late 1950s and early 1960s. Unraveling the teratogenic basis of thalidomide morphed into a more general investigation of its modes of action which have been attributed to anti-angiogenic, immunomodulatory, and anti-inflammatory properties. A resurgence of interest in thalidomide for various non-hematologic and hematologic conditions ultimately led to its FDA approval for erythema nodosum leprosum and multiple myeloma (MM) and the approval of its derivative lenalidomide for MM and myelodysplastic syndrome with the del (5q) chromosome abnormality.
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disorder primarily related to mutations in activin-like receptor kinase-1 (ALK1) and endoglin (ENG), which serve as receptors for the transforming growth factor-beta (TGF-β) family of ligands. In the normal state, endothelial cell-pericyte contact and vessel stabilization are maintained via a feedback loop involving TGF-β1 signaling through endoglin and Alk-1. In HHT, disruption of this signaling loop is felt to contribute to excessive angiogenesis and, in turn, phenotypic hallmarks of the disease: telangiectasias and arteriovenous malformations (AVMs). Severe epistaxis and gastrointestinal bleeding leading to anemia are common, and life-threatening internal bleeding from AVMs may result in substantial morbidity or death.
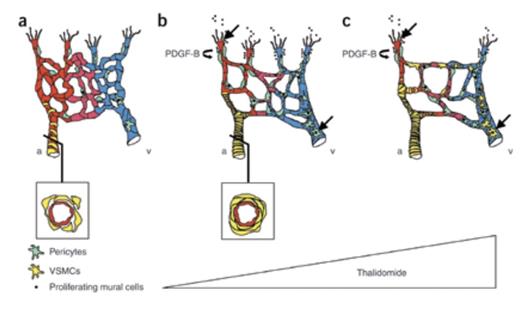
Schematic Illustration of How Thalidomide Normalizes Vascular Malformations in HHT. a) Reduced expression of endoglin or ALK-1 in endothelial cells reduces pericyte recruitment. Smooth muscle cell coverage of arteries is irregular, and vessels show endothelial hyperplasia and irregular capillary diameter. b) A low dose of thalidomide normalizes vessel coverage defects and excessive angiogenesis by stimulating mural cell recruitment. Mechanisms of thalidomide-induced vessel maturation include increased PDGF-B expression from the endothelial tip cells, which attracts co-migrating mural cells, and direct stimulation of mural cell proliferation. c) A high dose of thalidomide enhances the number of mural cells expressing α-SMA, indicating vessel maturity, and inhibits angiogenesis. Black arrows indicate that thalidomide targets both endothelial tip cells and mural cells directly to control vessel growth and maturation. a = artery; v = vein. Schematic illustration of how thalidomide normalizes vascular malformations in HHT.Figure Reprinted with permission from Macmillan Publishers Ltd: Nature Medicine (Lebrin F, Srun S, Raymond R, et al. Nat Med. 2010;16:420-428.) Copyright 2010.
Schematic Illustration of How Thalidomide Normalizes Vascular Malformations in HHT. a) Reduced expression of endoglin or ALK-1 in endothelial cells reduces pericyte recruitment. Smooth muscle cell coverage of arteries is irregular, and vessels show endothelial hyperplasia and irregular capillary diameter. b) A low dose of thalidomide normalizes vessel coverage defects and excessive angiogenesis by stimulating mural cell recruitment. Mechanisms of thalidomide-induced vessel maturation include increased PDGF-B expression from the endothelial tip cells, which attracts co-migrating mural cells, and direct stimulation of mural cell proliferation. c) A high dose of thalidomide enhances the number of mural cells expressing α-SMA, indicating vessel maturity, and inhibits angiogenesis. Black arrows indicate that thalidomide targets both endothelial tip cells and mural cells directly to control vessel growth and maturation. a = artery; v = vein. Schematic illustration of how thalidomide normalizes vascular malformations in HHT.Figure Reprinted with permission from Macmillan Publishers Ltd: Nature Medicine (Lebrin F, Srun S, Raymond R, et al. Nat Med. 2010;16:420-428.) Copyright 2010.
Lebrin and colleagues from the laboratories of Anne Eichmann in Paris and Christine Mummery in Leiden have exploited HHT as a disease model to “reveal” the “circumstances” by which thalidomide exerts its anti-angiogenic effects. First, they showed that thalidomide could reduce the frequency of epistaxis and increase hemoglobin levels in a small cohort of HHT patients. In an experimental model of HHT, which employed the neonatal retinas of mice heterozygous for a null mutation in ENG, a dose-dependent effect of thalidomide was observed; relatively lower doses normalized excessive vessel sprouting, while higher doses markedly reduced angiogenesis. Thalidomide also promoted retinal vessel maturation by stimulating recruitment of pericytes and vascular smooth muscle cells to the vascular plexus. Thalidomide up-regulation of endothelial cell-derived, platelet-derived growth factor beta (PDGFβ) was identified as a likely paracrine mechanism for recruitment of pericytes, which express the PDGFβ receptor (PDGFRβ). In support of this hypothesis, they showed that inhibition of PDGFRβ signaling with imatinib counteracted thalidomide’s effects on angiogenesis and on pericyte coverage of blood vessel endothelial cells. Thalidomide also exhibited direct effects on mural cell proliferation and behavior (e.g., development of membrane protrusions and branching extensions) that were independent of PDGFβ. In comparison to untreated HHT patients, the nasal mucosal skin biopsy of an HHT patient treated with thalidomide (100 mg daily) revealed increased numbers of smooth muscle layers surrounding blood vessels, recapitulating findings from the ENG-deficient mice.
In Brief
This study highlights the ability of thalidomide to reduce epistaxis in patients with HHT by promoting blood vessel maturation. In this experimental model of HHT, thalidomide’s modes of action reflect direct and indirect effects on pericyte recruitment and activation rather than a primary effect on vascular endothelial cells. While the conventional wisdom regarding thalidomide’s anti-angiogenic mechanisms may now need to be broadened, it is not clear whether the drug’s known ability to interfere with tumor growth is similarly related to enhancement of vessel maturation. Nonetheless, development of therapies targeting vessel stability may prove fruitful for patients with malignancies or for conditions related to vascular malformations, such as angiodysplasia and HHT. These mechanistic data also merit a careful appraisal of the utility of anti-angiogenic drugs in these disorders, since small-molecule multi-kinase inhibitors, which target PDGFRβ, may prove detrimental.
Competing Interests
Dr. Gotlib indicated no relevant conflicts of interest.