The investigators developed transgenic mice that delete the gene dicer only in osteoblast progenitor cells, which express osterix (Osx). Although dicer, which encodes a critical enzyme in the formation of microRNAs, was not deleted from the hematopoietic cells, these mice developed a form of myelodysplastic disease (MDS) with ineffective hematopoiesis and dysmorphic hematopoietic cells. In spite of normal cellularity and HSC numbers in the BM, the mice developed leukopenia with variable levels of anemia and thrombocytopenia. Hematopoietic progenitor cells showed increased apoptosis, and, morphologically, megakaryocytes were small and hypolobated, platelets enlarged, and granulocytes hypersegmented. Two percent of the mice developed a leukemia resembling AML M4 preceded by facial myeloid sarcomas. Of note, these leukemias expressed normal levels of dicer but were found to have abnormal genetics, proving that AML occurred in response to the microenvironment and not because of cell intrinsic deletion of dicer within the myeloid cells.
Both by in vitro co-culture of hematopoietic cells with dicer-deficient osteoprogenitor cells and by in vivo deletion of dicer specifically in more differentiated (osteocalcin positive) osteoblasts, the investigators proved that the induction of MDS is stage-specific to osteoblast progenitor cells.
To identify the mechanism by which dicer deletion in osteoblast progenitors may cause MDS, the investigators performed gene expression analysis. Differentially expressed genes and pathways included cytokines and stress response pathways, including significant down-regulation of the Shwachman–Bodian–Diamond (sbds) gene, which is linked to the human Shwachman–Diamond syndrome. Although the mechanism by which dicer deletion is linked to sbds expression is not clear, the investigators showed that knockdown of just the Sbds gene in osteoblast progenitors reproduces much of the MDS phenotype.
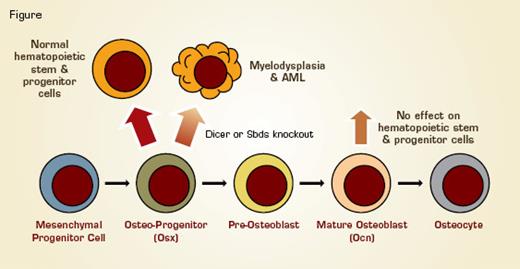
Osterix (Osx)-Expressing Osteoblastic Progenitor Cells Support Hematopoietic Cell Growth and Differentiation. When osteoblastic progenitor cells are induced to delete either the sbds (associated with Shwachman–Bodian–Diamond syndrome) or dicer (necessary for microRNA formation) gene in mice, the mice develop a form of myelodysplasia that can progress to AML. In contrast, when these genes are deleted only in mature osteocalcin (Ocn)-expressing osteoblasts, there is no effect on hematopoiesis.
Osterix (Osx)-Expressing Osteoblastic Progenitor Cells Support Hematopoietic Cell Growth and Differentiation. When osteoblastic progenitor cells are induced to delete either the sbds (associated with Shwachman–Bodian–Diamond syndrome) or dicer (necessary for microRNA formation) gene in mice, the mice develop a form of myelodysplasia that can progress to AML. In contrast, when these genes are deleted only in mature osteocalcin (Ocn)-expressing osteoblasts, there is no effect on hematopoiesis.
In Brief
This paper complements, in a very elegant way, prior data showing not only that the BM microenvironment and stem cell niche support physiologic hematopoiesis, but that alterations of the microenvironment, such as decreased expression of the retinoblastoma gene in cells of the BM microenvironment,1 can induce disease. It is particularly interesting that genetic alterations are induced within hematopoietic cells. Although this study does not prove that osteocytic lineage cells in the BM are directly involved in malignant processes in people, there are several lines of evidence that may point to abnormal BM niches in leukemia. For example, in patients with leukemia, there is decreased normal hematopoietic cell function even before the marrow has been entirely populated with leukemic cells, and, in CML, the marrow stromal cells also have the bcr-abl fusion protein. These data pose interesting questions for how we treat hematologic malignancies, and in particular for the field of HSC transplantation and whether increased attention to the BM microenvironment could improve treatment outcomes.
References
Competing Interests
Drs. Halene and Krause indicated no relevant conflicts of interest.