There is no trouble generating enthusiasm for treatment of β-thalassemia using gene therapy. The molecular basis of the disease is understood in great detail as is the structure and function of the normal gene, and β-hemoglobinopathies are the most prevalent inherited diseases, affecting millions worldwide. But a number of daunting technical obstacles, including the requirement for massive hemoglobin production in a lineage-specific manner and the lack of selective advantage for corrected hematopoietic stems cells, have restrained progress in the field. Now a multi-national group of investigators, led by Philippe Leboulch, has reported the first successful gene therapy for the disease in an 18-year-old male patient with transfusion-dependent βE/β0-thalassemia (the most common form of severe thalassemia in South East Asia). The patient’s CD34+ bone marrow cells were transduced ex vivo with a lentiviral vector construct that incorporated the β-globin locus control region and the β-globin gene (Figure). After conditioning with busulfan, he was transplanted with the gene-modified cells, and hematopoietic reconstitution was uneventful. After 33 months, he remains transfusion-independent with hemoglobin concentrations in the range of 9-10 g/dL. But the success of the procedure came with a twist as characterization of the chromosomal integration site of the vector indicated that most of the therapeutic benefit resulted from expansion of a stem/progenitor cell clone in which the integrated vector caused ectopic expression of the architectural transcription factor HMGA2 (Figure). While both granulocytes/monocytes and erythroblasts were HMGA2 integration-site positive (HMGA2 IS+), HMGA2 expression was observed only in erythroblasts, demonstrating that the HMGA2 IS+ cells were descendants of a primitive hematopoietic cell, but only the erythroid progeny expressed the chimeric gene. This latter finding is consistent with the erythroid-specific nature of the lentiviral vector construct (Figure).
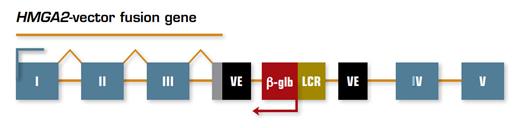
Schematic Representation of Integration of the Lentiviral Vector Construct into the HMGA2 Locus. The viral vector inserted into intron III of the HMGA2 gene, creating a fusion gene (shown as a line above the rectangles) that includes the first three exons of HMGA2 (shown as numbered gray boxes) and viral vector elements (VE) containing a cryptic splice site and the polyadenylation site (gray rectangle). The fusion mRNA transcript lacks exons IV and V of HMGA2 and is therefore missing binding sites for let-7 microRNAs that normally control protein expression. The bent arrows indicate the transcriptional direction of the fusion gene and β-globin genes.
Schematic Representation of Integration of the Lentiviral Vector Construct into the HMGA2 Locus. The viral vector inserted into intron III of the HMGA2 gene, creating a fusion gene (shown as a line above the rectangles) that includes the first three exons of HMGA2 (shown as numbered gray boxes) and viral vector elements (VE) containing a cryptic splice site and the polyadenylation site (gray rectangle). The fusion mRNA transcript lacks exons IV and V of HMGA2 and is therefore missing binding sites for let-7 microRNAs that normally control protein expression. The bent arrows indicate the transcriptional direction of the fusion gene and β-globin genes.
In Brief
This paper is important as proof-of-principle that a disease of β-globin can be treated successfully with gene therapy, thereby providing an approach to management of both β-thalassemias and sickle cell disease. It also provides unanticipated insight into how the lack of a selective advantage for transduced cells can be overcome. In this case, the growth advantage derives from integration of the vector into the HMGA2 locus resulting in erythroid-specific expression of a chimeric protein that putatively preserves the functional activity of HMGA2 (Figure). Nature has experimented with HMGA2 in a number of interesting settings. Ectopic expression of HMGA2 as a consequence of chromosomal translocation underlies a variety of benign mesenchymal tumors, and aberrant expression due to gene rearrangement was reported to account for clonal expansion of hematopoiesis in two patients with paroxysmal nocturnal hemoglobinuria,1 one of whom has been followed for 17 years without evidence of leukemic transformation. These observations support the concept of benign clonal hematopoiesis and suggest that ectopic expression of genes that provide a growth/survival advantage may be exploited for therapeutic purposes without risk of malignant transformation. It will thus be of great interest to identify the gene targets of HMGA2 and to determine mechanisms by which HMGA2 can be activated without gene disruption. As the saga unfolds, expect Mother Nature to keep things lively with a few more tantalizing twists, just the way we like it.
References
Competing Interests
Dr. Parker indicated no relevant conflicts of interest.