The immediate reaction to the diagnosis and treatment of Hodgkin lymphoma (HL) is the exhilaration of an 80 percent overall cure rate, which is then tempered by the anxiety of not being able to predict if your patient will be among the minority with recurrent and fatal disease. Therapy of HL is based on Ann Arbor staging to define limited- or advanced-disease groups. In advanced-disease settings, risk stratification is done using the seven prognostic factors of the International Prognostic Score (IPS). Uncertainty remains, however, regarding which patients will do poorly with conventional treatment and which patients might be cured with limited exposure to therapies that can induce late complications such as secondary malignancies and accelerated heart disease.
Steidl et al. from the British Columbia Cancer Agency report the results of a retrospective, multicenter study that utilized immunohistochemistry (IHC) and gene expression profiling to determine which cellular and microenvironmental signals within lymph node (LN) biopsy samples obtained at the time of diagnosis of classic HL correlated with treatment failure. Initial analyses of RNA isolated from 130 frozen specimens identified increased gene signatures of tumor-infiltrating macrophages, angiogenic cells, adipocytes, and Reed-Sternberg (RS) cells, along with overexpression of matrix metallopeptidases and underexpression of gene signatures for germinal center B cells as significantly associated with primary treatment failure. A validation study of 166 additional cases using IHC showed that increased numbers of CD68+ macrophages (≥ 5 percent) correlated most strongly with shortened progression-free survival (PFS) and increased likelihood of relapse after autologous hematopoietic stem cell transplantation. Conversely, a 100 percent disease-specific survival rate was observed for patients with limited-stage disease (stages IA and IIA) and absence of tumor-infiltrating macrophages. Reduced background small B cells (≤ 10 percent) and overexpression of MMP11 were also associated with shorter PFS. In multivariate analyses, CD68+ macrophages remained an independent adverse prognostic factor for disease-specific survival, outperforming the IPS.
In Brief
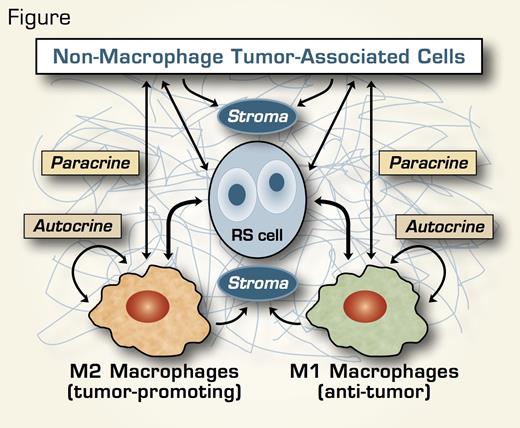
These clinicopathological correlations spotlighting tumor-infiltrating CD68+ macrophages in classic HL reinforce the critical roles of microenvironmental and immunomodulatory interactions between RS and non-tumor cells, and stromal, cytokine, and membrane molecules in disease pathobiology and treatment responsiveness. Tumor-associated macrophages (TAMs) and/or their cellular/stromal product signals have been implicated in improved survival in large B-cell lymphomas,1 poor outcome in follicular lymphoma (FL),2 or a variable response in FL depending on the number and activation status of the macrophages.3 Similarly, a recent study of pretreatment LN tissue in classic HL observed superior failure-free survival rates with overexpression of macrophage activation genes,4 suggesting that the net influence of TAMs on prognosis and treatment response likely is a product of the relative contributions of classically activated M1 macrophage phenotypes (with antitumor activity) versus alternatively activated M2 phenotypes (with immunosuppressive and/or tumor promoting properties) (Figure). If additional IHC studies confirm the value of CD68+ TAMs in classic HL as a prognostic biomarker, this readily available parameter will provide a powerful new tool for risk stratification and therapeutic decision-making for patients with both limited- and advanced-stage disease.
References
Competing Interests
Drs. Rosinski and Linenberger indicated no relevant conflicts of interest.