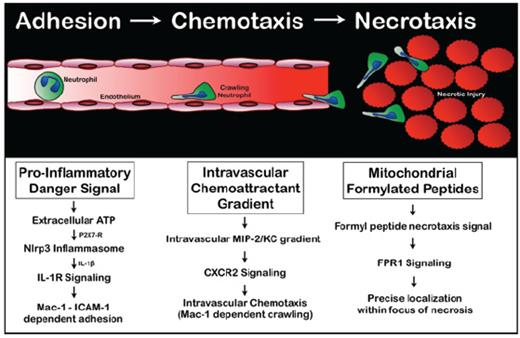
A murine model of focal hepatic necrosis was induced by localized thermal injury on the surface of the liver, followed by subsequent use of spinning disk video confocal microscopy to visualize the response of fluorescence-tagged neutrophils. Neutrophils adhered to the microvascular endothelium via leukocyte integrin Mac1 binding to endothelial ICAM-1 and accumulated within the area of necrosis. There was directional chemotaxis toward the necrotic tissue, ultimately infiltrating the area of cell death. Remarkably, the majority of neutrophils migrated via the intravascular route, which was often a less-direct course than transmigrating out of the vessel into the tissue. Purinergic danger signals such as ATP released from damaged cells directed neutrophils to sites of necrosis by inducing cytokine production and/or promoting leukocyte migration (Figure).
Administration of an exogenous ATPase (apyrase) resulted in a marked reduction in neutrophil recruitment but did not inhibit the ability of recruited neutrophils to migrate toward the focus of injury. Extracellular ATP, via P2X7 receptor signaling, activates the Nlrp3 inflammasome, including inflammatory cytokines such as interleukin (IL)-1b. Selective inhibition of P2X7 receptors resulted in reduced neutrophil recruitment in response to tissue injury. The CXCL2 chemokine, macrophage inflammatory protein 2 (MIP-2), immobilized on the luminal endothelial surface directed intravascular chemotaxis toward foci of damage, confirming that neutrophil chemotaxis is directed by a functional gradient of intravascular chemokines. The intravascular gradient of MIP-2 was consistently observed to abruptly end at the border of necrotic tissue. It was hypothesized that necrotic cells released an independent chemo-attractant or “necrotaxis” signal, which directs neutrophil migration beyond the intravascular chemokine gradient. Necrotic cells release damage-associated molecular patterns or DAMPs, including formylated peptides from mitochondria. In vitro, blockade of neutrophil formyl-peptide receptor 1 (FPR1) with inhibitory antibodies or the selective antagonist cyclosporine H (CsH) significantly attenuated neutrophil chemotaxis toward necrotic cells. FPR1 signals controlled only directionality within the necrotaxis zone.
In Brief
The authors conclude that intravascular danger sensing and recruitment mechanisms have evolved to limit collateral damage during responses to sterile injury by allowing neutrophils to migrate intravascularly as they navigate through healthy tissue to sites of injury. Necrotaxis signals promote localization of neutrophils directly into existing areas of injury to focus the innate immune response on damaged areas and away from healthy tissue, providing an additional safeguard against collateral damage during sterile inflammatory responses. This allows the innate immune system to clean up the dead without killing the living. Observing nature, as Celsus advised, really does provide us with the clues to heal. To really appreciate these findings check out the videos in the paper at: