A lingering question in the study of myeloproliferative neoplasms (MPNs) is how the singular JAK2 V617F mutation results in distinct clinical presentations. The mutation is identified in more than 95 percent of patients with polycythemia vera (PV) and 50-60 percent of patients with essential thrombocythemia (ET) and primary myelofibrosis. In addition to host genetic modifiers, higher JAK2 V617F allele burden and/or homozgyosity for mutant JAK2 in PV compared to ET has been invoked as one mechanistic basis for this difference in phenotypic diversity. However, the application of several experimental techniques has not yet convincingly demonstrated biologic differences in the activated signaling pathways downstream of JAK2 V617F between PV and ET. Such results may be partly confounded by analyses of samples intermixed with varying proportions of wild-type (WT) and mutant JAK2 clones, as well as other patient variables.
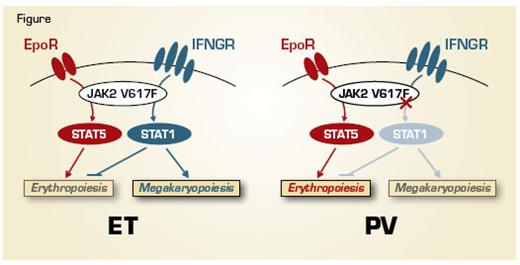
In order to sidestep this “clonal confusion,” Dr. Chen and colleagues from Anthony Green’s laboratory in the U.K. cultured and genotyped thousands of erythroid colonies from PV and ET patients, each derived from a WT or mutant JAK2 erythroid progenitor cell. Pooled WT and heterozygous JAK2 V617F erythroid colonies were subjected to biologic assays, including expression arrays, flowcytometry, and immunocytochemistry. Expression profiling revealed the surprising finding that JAK2 mutant colonies were more closely related to WT colonies from the same individuals than to mutant colonies from other patients. The profiling also revealed that interferon (IFN)-regulated genes were significantly up-regulated in ET compared to PV mutant erythroblasts. Increased expression of phosphorylated STAT1 was identified in ET, but not PV erythroblasts, a finding consistent with its essential role in IFNγ receptor signaling. Further, the expression of constitutively active STAT1 in K562 cells or CD34+ cord blood cells enhanced megakaryocytic differentiation and reduced erythroid differentiation. Conversely, inhibition of STAT1 signaling via introduction of a dominant negative form of STAT1 in CD34+ progenitors from ET patients resulted in a PV-like phenotype, with increased numbers of erythroid clones and reduced numbers of megakaryocytic clones.
In Brief
These data highlight the power of using clonal analysis to unmask differences in intracellular signaling that arises from a specific somatic mutation in related diseases. Preferential activation of STAT1 constrains erythroid differentiation and promotes megakaryocytic development, leading to an ET phenotype (Figure). In contrast, a reduced phospho-STAT1 response to JAK2 V617F promotes erythroid differentiation, as observed in PV. The finding that JAK2 V617F induces only minor effects on global gene expression (compared to WT JAK2) within the same patients serves to highlight the relative importance of inter-patient genetic variation as a contributor to disease diversity. This study raises several important questions, including what the biologic basis is for differential activation of STAT1 in ET versus PV, and how the marked differences in interferon signaling between PV and ET patients can be reconciled with the beneficial effects of interferon-α in both MPN subtypes. It will be of interest to evaluate whether changes in STAT1 activation herald the dynamic evolution between ET and PV observed in some patients.
Competing Interests
Dr. Gotlib indicated no relevant conflicts of interest.