Dr. Flaumenhaft indicated no relevant conflicts of interest.
The underlying molecular events responsible for antiphospholipid antibody syndrome (APS) have been difficult to pinpoint. Characterization of causative antibodies has demonstrated that they are not actually directed at phospholipids, but rather directed at plasma proteins that bind phospholipids. β2-glycoprotein I (β2-GPI) is an important antigen in this regard, but despite its high plasma concentration and central role in APS, the function of β2-GPI in normal physiology remains poorly defined. One function that has been observed is the binding of dimerized β2-GPI to members of the LDL receptor family on endothelial cells, yet the significance of this interaction is unclear. A group led by Dr. Chieko Mineo at Southwestern Medical Center now describes an antiphospholipid antibody-mediated signaling pathway involving β2-GPI and apolipoprotein E receptor 2 (apoER2) that leads to inhibition of endothelial NO synthetase (eNOS). This pathway induces leukocyte-endothelial interactions and thrombus formation in vivo.
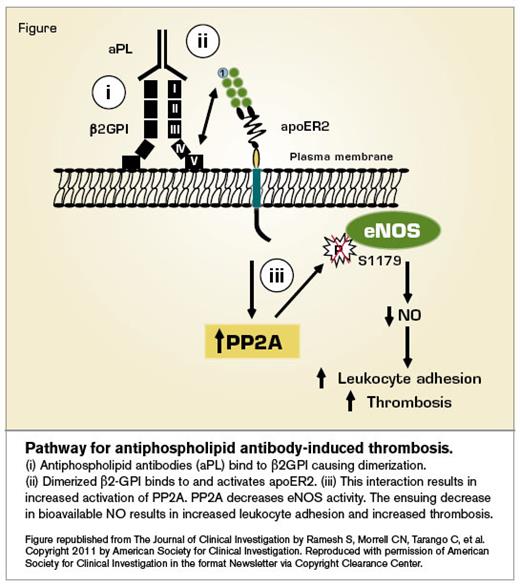
Pathway for antiphospholipid antibody-induced thrombosis.
Pathway for Antiphospholipid Antibody-Induced Thrombosis
Pathway for Antiphospholipid Antibody-Induced Thrombosis
The investigators found that incubating cultured endothelial cells with antiphospholipid antibodies isolated from patients with APS led to decreased eNOS activity and increased adhesion of monocytes. Their studies demonstrated a pathway in which dimerization of β2-GPI by antiphospholipid antibodies enabled binding to and activation of apoER2, leading to activation of the phosphatase PP2A. PP2A then elicited dephosphorylation of eNOS, with a resulting decrease in NO production and increase in leukocyte-endothelial cell interactions (Figure). The investigators performed several experiments to demonstrate that this pathway was operative in vivo as well. They used intravital microscopy to demonstrate that the leukocytes adhered to the endothelium following infusion of antiphospholipid antibodies into mice, noting that antiphospholipid antibody-mediated leukocyte adhesion was impaired in mice lacking eNOS or ApoER2. Thrombus formation was also evaluated, and the reduction in closure time following infusion of antiphospholipid antibodies was absent in mice lacking eNOS or ApoER2, indicating a role for this pathway in antiphospholipid antibody-mediated thrombosis.
In Brief
The prevalence of antiphospholipid antibodies in the general population is approximately 1 to 5 percent. These antibodies can contribute to both venous and arterial thrombosis as well as recurrent pregnancy loss. How these antibodies elicit vascular disease has been a recondite puzzle, and, although some pieces of the puzzle have been identified during the past three decades, a coherent picture of the molecular mechanism by which they elicit thrombus formation has been slow to emerge. The article by Dr. Ramesh and colleagues now fits these pieces together into a cohesive pathway. While the β2-GPI/ApoER2-dependent eNOS inactivation by antiphospholipid antibodies may not be the only operative pathway that mediates vascular disease in APS, the in vivo data indicate that it is an important one. Understanding the molecular mechanism of APS could redirect drug development programs to focus on more proximal events in the pathway, potentially leading to therapies with fewer side effects than traditional anticoagulation.
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.