It is well known to hematologists that managing patients who require chronic anticoagulation is a cumbersome task, and until very recently, warfarin, a vitamin K antagonist, has been the only available oral anticoagulant. Warfarin treatment requires regular monitoring with prothrombin time testing, and, since it has multiple interactions with food and drugs, frequent dose adjustments are necessary. Despite regular monitoring by either patient self-management or hospital-based anticoagulation clinics, patients fall outside the desired therapeutic range about one-third of the time. So the recent FDA approval of the orally active direct thrombin inhibitor (DTI) dabigatran etexilate has been welcome news.
DTIs are a new class of anticoagulants that function by direct interaction with the active site of thrombin and do not require the natural physiological inhibitor antithrombin, so they are mechanistically distinct from heparin, low-molecular-weight heparin, and fondaparinux (Figure). Three parenterally administered DTIs (lepirudin, bivalirudin, and argatroban) are currently used in the acute treatment of heparin-induced thrombocytopenia (HIT).
Dabigatran etexilate is the second oral DTI. The first, ximelagatran, was found to be effective as an antithrombotic in a variety of clinical situations. However, the drug was not approved by the FDA for concerns of associated hepatotoxicity and its distribution was discontinued in Europe. Dabigatran etexilate is a prodrug that is rapidly converted to its active metabolite, dabigatran, by a ubiquitous, non-specific plasma esterase, and reaches its peak plasma anticoagulant effect ~0.5-2 hours after oral administration.1 Drug clearance is completed primarily by the kidney, with a plasma half-life of ~12-17 hours. Metabolism of dabigatran does not involve the hepatic cytochrome P450 system, so it has minimal drug-drug and drug-food interactions and does not require regular monitoring, thus fulfilling the criteria for an oral anticoagulant to replace warfarin — rapid onset of action, ease of use, and no need for regular monitoring.
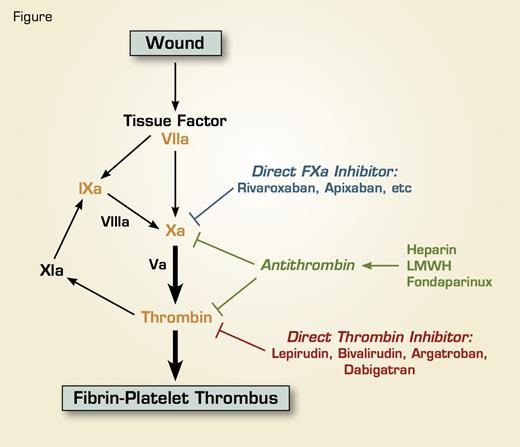
At the site of a vascular wound, tissue factor is exposed, which acts as a physiological cofactor for Factor VIIa, and the tissue factor/FVIIa complex activates the clotting cascade. Tissue factor/FVIIa activates factor X either directly or indirectly through the activation of factor IX, and the resultant factor Xa activates prothrombin to thrombin. Thrombin converts fibrinogen to fibrin and also activates platelets, resulting in the formation of a fibrin-platelet thrombus. Thrombin can also activate factor XI, and factor XIa in turn activates FIX, thus serving as a tertiary pathway of thrombin generation. Warfarin is a vitamin K antagonist and targets prothrombin, factor VII, factor IX, and factor X (Red), with factor X and prothrombin being the main targets. Heparin, lowmolecular-weight heparin (LMWH), and the synthetic pentasaccharide fondaparinux serve as anticoagulants by activating antithrombin, a plasma serine protease inhibitor that functions as the major physiological regulator of the clotting cascade; antithrombin then inhibits factor Xa and thrombin. Both the direct thrombin inhibitors and the direct factor Xa inhibitors inhibit their respective targets directly without the need of antithrombin.
At the site of a vascular wound, tissue factor is exposed, which acts as a physiological cofactor for Factor VIIa, and the tissue factor/FVIIa complex activates the clotting cascade. Tissue factor/FVIIa activates factor X either directly or indirectly through the activation of factor IX, and the resultant factor Xa activates prothrombin to thrombin. Thrombin converts fibrinogen to fibrin and also activates platelets, resulting in the formation of a fibrin-platelet thrombus. Thrombin can also activate factor XI, and factor XIa in turn activates FIX, thus serving as a tertiary pathway of thrombin generation. Warfarin is a vitamin K antagonist and targets prothrombin, factor VII, factor IX, and factor X (Red), with factor X and prothrombin being the main targets. Heparin, lowmolecular-weight heparin (LMWH), and the synthetic pentasaccharide fondaparinux serve as anticoagulants by activating antithrombin, a plasma serine protease inhibitor that functions as the major physiological regulator of the clotting cascade; antithrombin then inhibits factor Xa and thrombin. Both the direct thrombin inhibitors and the direct factor Xa inhibitors inhibit their respective targets directly without the need of antithrombin.
Dabigatran has been tested extensively for venous thromboembolism (VTE) prophylaxis in high-risk patients undergoing orthopedic surgery and has demonstrated either superiority or noninferiority to enoxaparin with no significant hepatotoxicity. Dabigatran is approved in Europe for VTE prophylaxis at an oral dose of 220 mg once daily, with the first dose given as a half-dose between one and four hours after surgery for a total of 10 days after total knee replacement and for 28-35 days after total hip replacement. For patients older than 75 years and those with creatinine clearance of 30-50 ml/minute, the total daily dose is reduced to 150 mg once daily. Dabigatran is contraindicated in patients with creatinine clearance less than 30 ml/minute.
The RE-LY trial addressed the efficacy of dabigatran versus warfarin to prevent stroke or systemic embolism in moderate- to high-risk patients with atrial fibrillation.2 Dabigatran dosed at 110 mg twice daily is equivalent to warfarin in efficacy but is associated with lower rates of major hemorrhage (2.7 % vs. 3.4 % per year), while dosing at 150 mg twice daily has a reduced stroke and systemic embolism rate (1.1 percent vs. 1.7 percent), with similar major hemorrhage rates. The FDA has approved dabigatran for this indication.
The RE-COVER trial showed that dabigatran (at 150 mg twice-daily dose) and warfarin (with INR targeted to 2-3) were equivalent for the treatment of acute VTE after an initial 5-10 day treatment with an injectable anticoagulant.3 A phase III trial testing the long-term efficacy of dabigatran in patients with recurrent VTE is ongoing, and, given the prior positive clinical trial results with ximelagatran, dabigatran is expected to be effective in the long-term treatment of VTE.
Patient convenience, safety, and clinical efficacy of dabigatran have now been demonstrated, but the treatment cost will likely be substantially higher than for warfarin. Based on the pricing in the United Kingdom and the data from the RE-LY trial, dabigatran has been estimated to be cost-effective compared to warfarin in the prevention of stroke in high-risk patients with atrial fibrillation.4 A similar conclusion has been reached in the use of dabigatran in VTE prophylaxis as compared to enoxaparin.5 Whether this applies to patients who require chronic anticoagulation for prevention of recurrent VTE remains to be seen.
Dabigatran, similar to the parenteral DTIs, does not have an effective antidote, and management of serious bleeding complications or acute reversal of anticoagulation for urgent surgery can be problematic. This remains a potential limitation for this new class of anticoagulants.
In addition to oral DTIs, oral factor Xa inhibitors have demonstrated clinical efficacy in prophylaxis against VTE in orthopedic surgery.6 Rivaroxaban has been approved in Canada and Europe for this indication. The anticipated approval of the oral factor Xa inhibitors should further broaden the armamentarium for anti-thrombotic therapy, and further competition may lead to more cost-effective treatment options for patients with VTE.