Division of Immunohematology and Tranfusion Medicine, Department of Oncology-Hematology, Ospedali Riuniti, Bergamo, Italy
Dr. Falanga serves on a Virtual Advisory Board for Pfizer and as part of the Registry Master Oncology at Sanofi-Aventis.
The close relationship between cancer and thrombosis has been known since the days of Armand Trousseau, who first described the clinical association between idiopathic venous thromboembolism (VTE) and occult malignancy in 1865. Today, we know that cancer is associated with a hypercoagulable state and a four-fold increase in thrombosis risk, with chemotherapy elevating this risk even more. Epidemiologic and population-based studies provide detailed information on the scale of the problem and the identification of VTE risk factors, including those related to the tumor (tumor type, clinical stage, chemotherapy, use of anti-angiogenic drugs or erythropoietic growth factors, and insertion of central venous catheters), and those related to individual patient characteristics (sex, race, age, previous VTE history, immobilization, and obesity). Thrombosis has a significant impact on the morbidity and mortality of cancer; therefore, it is important to identify which patients may be at higher risk than others, especially before starting chemo-radiotherapy or surgery.
As our knowledge of thrombosis risk accrues, more sophisticated methods of risk assessment are being developed. Prediction models for chemotherapyassociated VTE have become available and include many of the risk factors listed above as well as biological markers,1 such as leukocyte and platelet counts and circulating levels of tissue factor (TF), P-selectin, and D-dimer.2 The hope is that targete thromboprophylaxis utilizing predictive models may improve risk-benefit ratio. In this context emerges the important role of circulating cell-derived microparticles (MP), submicrometric membrane-bound vesicles that circulate in blood and can carry TF and procoagulant phospholipids on their surface. Variations in MP quantity and/or phenotype are relevant pathogenic markers of thrombosis and vascular damage and are associated with a higher risk of VTE in cancer patients. MP levels are currently under study as a criterion to enroll high-risk cancer patients in thromboprophylaxis trials.3
Recently, a number of guidelines for the prevention and management of VTE in cancer patients have been released from major American and European scientific societies. After an initial period of wide heterogeneity, a consensus is emerging concerning thromboprophylaxis in hospitalized non-surgical and surgical cancer patients and treatment of VTE in cancer patients,4 although controversies remain concerning duration of prophylaxis after surgery, use of thromboprophylaxis in ambulatory patients receiving anti-cancer therapies, strategies for treatment of VTE after the first six months of therapy with low-molecular-weight heparin, treatment of VTE recurrences, and the role of new oral anticoagulants.
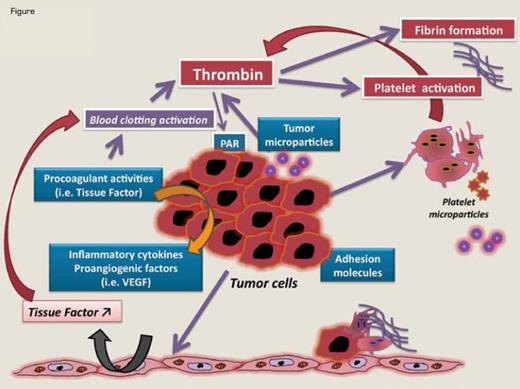
Mechanisms of Hemostatic System Activation by Tumor Cells Involves Different Hemostatic Pathways. Tumor cells produce procoagulant, fibrinolytic, and platelet-aggregating activities and release proinflammatory and proangiogenic cytokines and procoagulant microparticles. Tumor cells interact with host vascular and blood cells (i.e., platelets, leukocytes, and endothelial cells) by means of direct adhesion, which activates the prothrombotic properties of these cells. Tumor cell-derived TF plays a central role in the generation of thrombin, but TF can also contribute to tumor growth and metastasis by coagulation-independent mechanisms, including influencing the expression of VEGF by the malignant cells and vascular cells and activating the PAR-2 signaling.The generation of activated coagulation proteases (FVIIa, FXa, thrombin) and the formation of fibrin represent coagulation-dependent mechanisms of tumor progression, as they promote neo-angiogenesis and tumor proliferation. Fibrin coats also protect circulating cancer cells from attack by the host immune system.
Mechanisms of Hemostatic System Activation by Tumor Cells Involves Different Hemostatic Pathways. Tumor cells produce procoagulant, fibrinolytic, and platelet-aggregating activities and release proinflammatory and proangiogenic cytokines and procoagulant microparticles. Tumor cells interact with host vascular and blood cells (i.e., platelets, leukocytes, and endothelial cells) by means of direct adhesion, which activates the prothrombotic properties of these cells. Tumor cell-derived TF plays a central role in the generation of thrombin, but TF can also contribute to tumor growth and metastasis by coagulation-independent mechanisms, including influencing the expression of VEGF by the malignant cells and vascular cells and activating the PAR-2 signaling.The generation of activated coagulation proteases (FVIIa, FXa, thrombin) and the formation of fibrin represent coagulation-dependent mechanisms of tumor progression, as they promote neo-angiogenesis and tumor proliferation. Fibrin coats also protect circulating cancer cells from attack by the host immune system.
Critical for the design of appropriate pharmacologic interventions for cancer-associated VTE is a better understanding of multi-factorial mechanisms underlying the hypercoagulability. Among other factors, a prominent role is played by tumor cell-specific clot-promoting properties, which may also contribute to the process of tumor growth and dissemination.5 These include expression of TF by tumor cells, production of MP and inflammatory cytokines by tumor and/or host cells, and direct adhesion of tumor cells to platelets, leukocytes, and endothelial cells (Figure). TF of tumor origin is a key molecule that initiates blood clotting and also supports tumor growth and metastasis by coagulation-independent mechanisms, such as up-regulation of VEGF and activation of PAR-2. Coagulation system activation, with generation of thrombin and fibrin and activation of platelets, leukocytes, and endothelial cells, plays a crucial role in the progression of cancer. Recent extensive experimental evidence shows that platelets support tumor metastasis.6 Within the circulatory system, platelets (and fibrin) guard tumor cells from immune elimination and promote their arrest at the endothelium, favoring the establishment of secondary tumors. Experimental studies in mice demonstrate the role of platelet glycoproteins GPIbα, GPVI, and P-selectin in supporting this process.7 Moreover, platelets exert a protective role in the maintenance of tumor vascular integrity and may represent a target for the specific destabilization of tumor vessels.8 Finally, MP shed by tumor cells and platelets, which carry pro-angiogenic factors, are newly identified players in maintaining tumor growth.
In the last decade, the story was advanced by the discovery of a complex scenario in which oncogenic events drive the procoagulant conversion of tumor cells. Oncogene and tumor suppressor gene-mediated neoplastic transformation driven by activation of MET, loss of PTEN, induction of K-ras, and/or loss of p53 in several experimental models of human cancers has been associated with activation of clotting pathways as an integral feature of the transformation.5 Signaling pathways triggered by one or more of these genes can result in activation of blood coagulation and platelet function and/or suppression of fibrinolysis, which in some cases produced thrombosis and/or DIC in these models. Furthermore, a mutation of EGFR gene renders cancer cells hypersensitive to the action of coagulation proteins, such as TF; as a result, a microenvironment promoting tumor growth is generated.
In human malignancies, PML-RARα hybrid gene expression in patients with acute promyelocytic leukemia and JAK2V617F expression in patients with myeloproliferative neoplasms (MPNs) are associated with expression of a prothrombotic phenotype. Among other properties, platelets from JAK2-positive patients with MPN express increased TF on their membranes.9
With a better understanding of the molecular events associated with cancer thrombophilia, new targets for development of bifunctional drugs (i.e., those capable of attacking both the malignant process and the coagulopathy) may be identified. Perhaps one example of a targeted, bifunctional therapy is ATRA for treatment of APL. Until more specific targeted therapies are available, however, we must rely on anticoagulant drugs for prophylaxis and treatment of thrombosis. In this context, it is worth noting that anticoagulant treatments have been reported to improve survival in cancer patients.10 Given the limitations of the available studies, the routine use of anticoagulants as primary anti-cancer therapy cannot be recommended. Nevertheless, the data from meta-analyses may provide a stimulus to test the hypothesis in properly designed, large randomized clinical trials. Additional efforts to develop therapies that rapidly correct the hypercoagulable state of cancer are required. As the molecular basis becomes better elucidated, development of drugs that will target both the malignant process and the resultant hypercoagulability is a realistic goal.