This study from the laboratory of Dr. Joseph Italiano in Boston demonstrates that intact spectrin tetramers are critical for normal megakaryocyte maturation and platelet formation. While a critical role for spectrin is known for red blood cells and for platelet activation, it has not been shown previously that spectrin may also play a role in platelet biogenesis.
Spectrin is a cytoskeletal protein that supports cell structure by forming a latticework that is attached to the inner side of the plasma membrane. Humans have two alpha (α1, α2) and five beta (β1-5) spectrin genes. Mature tetrameric spectrin consists of two a/b spectrin dimers joined head to head. In red blood cells, α1 and β1 are the primary spectrins, and mutations in these erythroid spectrins cause RBC abnormalities including hereditary spherocytosis and elliptocytosis. While many nonhematopoietic cells, including neurons, express spectrins, they are usually non-erythroid spectrins. The investigators in this study demonstrated that megakaryocytes express α1 and α2 as well as β1 and β2 spectrins. However, the erythroid (α1, β1) spectrins are expressed at much lower levels, and their proportion decreases during platelet biogenesis suggesting that α2 and β2 are the key spectrins in platelets.
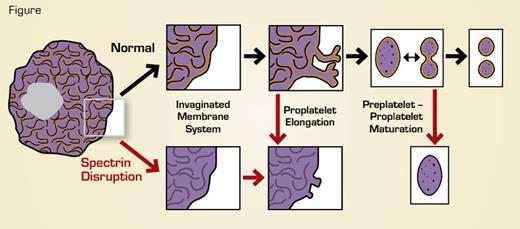
Disruption (red arrows) of spectrin tetramers disrupts multiple stages of megakaryocyte maturation and platelet formation. After disruption, megakaryocytes fail to form a normal invaginated membrane system, proplatelets that started to elongate retract, and proplatelet “barbells” lose their structure and fail to form platelets. Yellow indicates supporting spectrin skeleton.
Disruption (red arrows) of spectrin tetramers disrupts multiple stages of megakaryocyte maturation and platelet formation. After disruption, megakaryocytes fail to form a normal invaginated membrane system, proplatelets that started to elongate retract, and proplatelet “barbells” lose their structure and fail to form platelets. Yellow indicates supporting spectrin skeleton.
Using a novel spectrin tetramer-disrupting protein, Patel-Hett and colleagues demonstrated a requirement for intact spectrin tetramers at multiple stages of platelet biogenesis. Depending on when the disrupting reagent was introduced into the cells, effects were assessed on a) megakaryocyte cytoplasmic maturation, b) proplatelet extension, and c) transition from the proplatelet to the preplatelet to mature platelets (Figure). During megakaryocyte maturation, an extensive invaginated membrane system forms. Without intact spectrin tetramers, megakaryocytes lacked this extensive membrane structure. However, the alpha and dense granules, which are essential for functional platelets, did not seem to be affected. When tetramers were disrupted after megakaryocyte maturation, then effects on proplatelet extension could be assessed. Treated megakaryocytes showed far fewer proplatelet extensions, and those that were present were blunted. Similarly, when proplatelet/preplatelet interconversion was assessed using time-lapse microscopy, disruption of spectrin tetramers led to a dramatic change in cell shape, with the cell rounding up becoming unable to form/release mature platelets.
In Brief
The clinical implications of these findings are not yet clear. In general, patients with spectrinopathies have not been reported to have platelet function abnormalities. However, detailed platelet function studies have not been determined in a rigorous manner. In one often-cited report, Jarolim et al. present a case report of severe hemolysis and red cell fragmentation caused by the combination of a spectrin mutation with a thrombotic microangiopathy.1 Perhaps in such cases there are mutations in the non-erythroid spectrins that affect platelet biogenesis/function in addition to RBC structure.
References
Competing Interests
Dr. Krause indicated no relevant conflicts of interest.