Dr. Johnson is an author on this study.
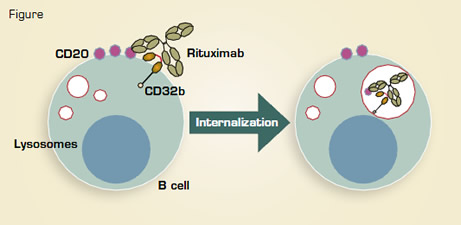
There is mounting evidence that engagement of CD20 on the surface of B cells by monoclonal antibodies may result in the disappearance of the antigen, an effect that has been reported as more marked with the type I reagents such as rituximab and ofatumumab than with the type II obinutuzumab (GA101) and tositumomab.1 This may be important, not only because disappearance of the antigen can impair binding of anti-CD20 to the target cell, but also because it appears to reduce the half-life of the antibody by internalization of the CD20:anti-CD20 complexes and their intracellular degradation.
Martin Glennie and Mark Cragg from the University of Southampton, U.K., have previously shown that this process is heterogeneous across disease types, occurring much more frequently in chronic lymphocytic leukemia and mantle cell lymphoma than in the germinal center types.1 The same group has now expanded these observations, and, having hypothesized that the effect might be mediated by Fc receptor binding on the target cells themselves, they went on to look at the dominant type of Fc receptor found on B cells — the inhibitory FcγRIIb (CD32b). Comparisons of the level of expression of CD32b with the degree of CD20 internalization on a variety of normal or malignant human B cells following rituximab treatment showed a clear correlation. This finding was reinforced by the prevention of internalization by a blocking anti-CD32b antibody and by comparing a CD32b-negative cell line to the same cells transfected with the FcγRIIb. This showed that those with the highest levels of expression underwent the most CD20 internalization. This process was accompanied by phosphorylation of the Fc receptor, an effect dependent mainly upon the presence of the Fc receptor on the same cells as the CD20, following which the complexes could be tracked to the B-cell lysosomes, where they were degraded.
The potential effects of this internalization were tested in an in vitro model of antibody treatment, where there was a markedly reduced level of macrophage phagocytosis following type I antibody, while the type II antibodies were largely unaffected. Once again, Fab fragments to CD32b were capable of reversing the fall in phagocytosis after rituximab treatment. Finally, the authors examined the relationship between the expression of CD32b and the outcomes of rituximab-based therapy in a small retrospective series of patients with mantle cell lymphoma. They found that the progression-free survival was significantly shorter among patients with high FcγRIIb expression on the lymphoma cells, as determined by immunohistochemistry.
It is becoming clear that engagement of various Fc receptors is a critical part of the mechanism of action for anti-CD20 antibodies, although previous attention focused on the stimulatory FcγRIIIa on host myeloid effector cells, following the observation that different polymorphisms correlated with responses.2 This paper looks at the FcγRIIb on the malignant B cells themselves and offers the possibility to distinguish those types that should maintain CD20 on the cell surface after rituximab binding from those in which the antigen is likely to be rapidly internalized and degraded. This, in turn, may prove useful either for selecting those illnesses in which a non-internalizing type II antibody may be preferable or for guiding combination treatment, perhaps using blocking antibodies to CD32b. Clearly the clinical findings need to be verified in a larger series of patients and ultimately tested in prospective trials, but they hold the possibility of a mechanism-based approach to anti-CD20 treatment now that the details of interaction on the cell surface are yielding their secrets.