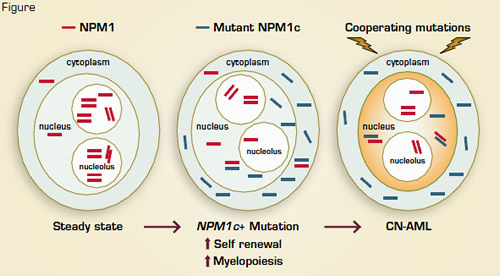
Acute myeloid leukemia (AML) represents a heterogeneous group of malignancies with great variability in clinical course and response to therapy. Mutations involving the nucleophosmin (NPM1) gene are found in one-third of all AML cases and roughly 60 percent of cytogenetically normal AML. NPM1, an oligomeric, nucleolar chaperone protein, normally shuttles molecular partners between the nucleolar, nucleoplasmic, and cytoplasmic compartments and also participates in nucleolar assembly, ribosome biogenesis, chromatin organization, centrosome duplication, nuclear mRNA maturation, and DNA repair. NPM1 mutations (referred to as NPM1c+) cause aberrant localization of NPM1c to the cytoplasm. AMLs with NPM1c+ characteristically exhibit low CD34 expression, FLT3-ITD mutations, a characteristic gene expression profile (e.g., HOX overexpression) and microRNA signature, multi-lineage involvement, and a relatively favorable prognosis (in the absence of FLT3-ITD). Clinical observations that NPM1c+ stably persists during AML relapses while associated genetic mutations may be lost or acquired suggest that mutant NPM1 is an important “founder” genetic lesion and may therefore be a valuable target for anti-leukemic therapy.
To better understand the molecular and cellular roles of NPM1c+ in AML pathobiology, Vassiliou and colleagues in the laboratory of Allan Bradley at Cambridge Institute for Medical Research studied a conditional knock-in mouse model that expressed the common, type A (humanized) form of NPM1c mutation. They observed that hematopoietic cells from the NPM1cA transgenic mice recapitulated the human phenotype with cytoplasmic NPM1c localization, differential expression of Hox and other genes, and expansion and increased self-renewal of myeloid progenitors. Some of these mice also developed late-onset AML, suggesting that additional genetic lesions facilitated leukemic outgrowth. A transposon mutagenesis approach was then employed to induce secondary, cooperative events. Roughly 80 percent of mice that co-expressed NPM1cA and the Sleeping Beauty transposon developed rapid-onset AML, and all leukemias had persistent Hox overexpression, implying continued dependence on NPM1c-induced mechanisms. Common insertion site analyses identified GM-CSF mutagenesis and activation in many cases along with exclusive integrations in Flt3 or Rasgrp1 and involvement by a number of genes not previously linked with NPM1c+ AMLs, including Nup98, Nf1, Bach2, and Dleu2.
Secondary Leukemogenic Mutations May Involve Alterations of Proliferation Signals, Transcription Factors, Other Signaling Molecules, and/or Tumor Suppressors.
Secondary Leukemogenic Mutations May Involve Alterations of Proliferation Signals, Transcription Factors, Other Signaling Molecules, and/or Tumor Suppressors.
In Brief
These data establish NPM1c+ as an initiator of myeloid cell transformation — an effect mediated, at least in part, by Hox gene overexpression and increased self renewal. Secondary leukemogenic mutations may involve alterations of proliferation signals (e.g., Flt3, as observed in human NPM1c+ cytogenetically normal AML), transcription factors (Bach2), other signaling molecules (Rasgrp1, Nf1), and/or tumor suppressors (Dleu2) (Figure). Understanding the foundational role of NPM1c+ in AML reinforces the notion that disabling NPM1c might interrupt the collaborative, downstream molecular pathways required for maintenance of the leukemic clone. Indeed, recent studies indicate that inhibition of NPM1 oligomerization and reduction of NPM1c and wild-type NPM1 levels induces apoptosis and sensitizes NPM1c+, Flt3-negative AML cells to cytotoxic agents.1 The residual, wildtype NPM1 in NPM1c+ AML cells might therefore provide an additional Achilles heel for therapeutic exploitation. Together, the observations by Vassiliou et al. provide key information on cytogenetically normal AML development, and they illustrate the utility of a rationally designed, “humanized” murine transgenic model to characterize the multi-step pathogenesis of human cancer.
References
Competing Interests
Drs. Ostronoff and Linenberger indicated no relevant conflicts of interest.Vassiliou GS, Cooper JL, Rad R, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in miceMutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43:470-475.