Glanzmann thrombasthenia (GT) is an autosomal recessive bleeding disorder with an incidence of ~1:1,000,000 that was first reported by Edward Glanzmann in 1918. It results from mutations in genes encoding the heterodimeric αIIbβ3 complex on platelets. More than 170 genetic abnormalities have been described that produce the clinical phenotype. Because expression of αIIb and β3 are co-dependent, a defect in expression of one component of the heterodimeric complex leads to subnormal expression of the other component. Fibrinogen binds αIIbβ3 and bridges platelets, leading to platelet aggregation. GT is associated with a platelet aggregation defect and mucocutaneous bleeding, which can be life-threatening. Patients with GT are treated with platelet transfusions and non-specific measures, including anti-fibrinolytics and recombinant factor VIIa. Patients frequently become refractory to platelet transfusions due to the development of anti-platelet antibodies. Allogeneic bone marrow transplantation can be curative but insufficient donor availability and procedure-associated morbidity and mortality complicate treatment.
Great Pyrenees dogs can have a severe bleeding diathesis due to GT caused by a splicing defect in the αIIb gene.1 Fang et al. in the laboratory of David Wilcox in the Department of Pediatrics at the Medical College of Wisconsin and the Blood Research Institute at the Blood Center of Wisconsin now report treatment of three dogs with GT by autologous transplantation of αIIb gene-corrected hematopoietic stem cells (HSCs). In these studies, HSCs were mobilized from bone marrow into peripheral blood by treating the dogs with the cytokines, granulocyte-colony stimulating factor (G CSF), and stem cell factor (SCF). CD34+ cells, which are enriched in HSCs, were isolated from G-CSF/SCF-mobilized peripheral blood. The human αIIb gene was introduced into HSCs ex vivo using a lentiviral vector derived from the HIV-1 virus. Lentiviral vectors are attractive in gene therapy because they efficiently transduce both dividing and non-dividing cells; produce long-term, stable transgene expression; and are less immunogenic than other vectors. Currently, lentiviral vectors are being evaluated in 40 human gene therapy clinical trials for a variety of hematologic and non-hematologic disorders, including β-thalassemia and adrenoleukodystrophy.2
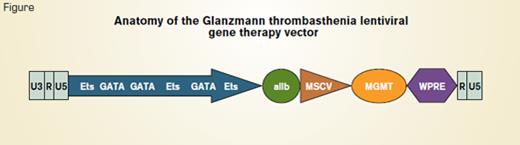
Anatomy of the Glanzmann Thrombasthenia Lentiviral Gene Therapy Vector
Anatomy of the Glanzmann Thrombasthenia Lentiviral Gene Therapy Vector
As a safety feature, Fang et al. used a self-inactivating (SIN) lentivirus that was produced by removal of the U3 enhancer/promoter from the 3’ viral long-terminal repeat (Figure). The 5’ long-terminal repeat of HIV-1 consisting of U3, R, and U5 elements was left intact. The αIIb gene promoter containing binding sites for the GATA and Ets transcription factors was used to target transcription specifically to megakaryocytes. Additionally, a cDNA encoding expression of a drug-resistant protein, P140K methylguanine methyltransferase (MGMT) under control of the murine stem cell virus promoter (MSCV), was added to allow for in vivo enrichment of lentivirus-transduced HSCs. The woodchuck hepatitis post-transcriptional regulatory element (WPRE) was included to enhance the efficiency of transgene expression. Nonmyeloablative pretransplant conditioning using either low-dose total body irradiation or busulfan was used to create a niche in the bone marrow for the transplanted cells to engraft.
Flow cytometry revealed that an average of 5,000 αIIbβ3 molecules was expressed on platelets from transplanted dogs. In contrast, normal canine platelets contain ~80,000 αIIbβ3 molecules. After transplantation, the dogs received O6-benzylguanine and carmustine to allow enrichment of genetically modified cells containing P140K MGMT. This treatment led to an increase in the number of platelets that expressed αIIbβ3 to ~10 percent of the total platelet population. Despite the relatively low density of αIIbβ3, platelet aggregation, clot retraction, platelet fibrinogen binding, and buccal mucosa, bleeding times were corrected in all three dogs. One of the dogs developed high-affinity anti-IIb antibodies that blocked platelet aggregation. The antibody titer decreased but remained measurable after treatment with intravenous immunoglobulin (IVIG). The dogs remained well for two, four, and five years after the gene therapy procedure with no evidence of hematologic or other abnormalities associated with vector insertional mutagenesis.
In Brief
The results of the study by Fang et al. demonstrate the feasibility of gene therapy for GT in a large animal. Relatively low-density expression of platelet αIIbβ3 compared with that of normal dogs sufficed to correct the hemostatic abnormalities associated with GT. The development of anti-IIb antibodies in one of the dogs was a significant complication and suggests that immunosuppression may be a necessary adjunct to gene therapy of GT in humans.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.