Director and Chair, Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research, Departments of Medicine and Cell Biology, Albert Einstein College of Medicine, Bronx, NY
There has been a keen interest over the past few years in understanding the cellular and molecular constituents of the stem cell niche. The notion that specific microenvironments could support hematopoietic stem cell (HSC) self-renewal and differentiation dates back several decades, but the exact nature of the niche has remained enigmatic. The conceptual framework of a stem cell niche, supported by experimental work in germline stem cells from C. elegans, has suggested that stem cells interact with a specific nurturing niche cell. Whether this idea holds true for the mammalian HSCs remains a matter of intense investigation and debate. Recent studies point, in fact, to an integrated multicellular complex that orchestrates HSC behavior and likely influences other microenvironments dedicated to committed, lineage-restricted progenitors.
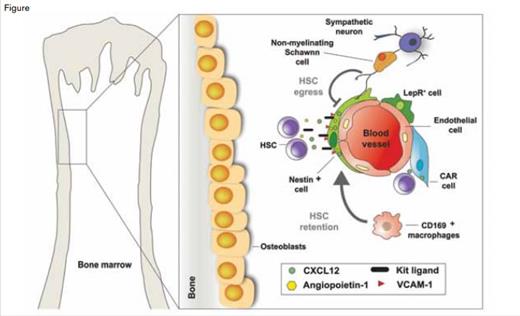
Schematic Representation Illustrating the Major Cellular Constituents of the Bone Marrow HSC Niche. A variety of cells, including osteoblasts, Cxcl12-abundant reticular (CAR) cells, Nestin+ mesenchymal stem cells (MSC), Leptin receptor (Lepr)-expressing perivascular cells, and endothelial cells, have been reported as possible components of the niche. Schwann cells, wrapping sympathetic nerve fibers, promote HSC quiescence. In total, the structural makeup of these various cells provides a specialized microenvironment regulating HSC self-renewal and differentiation, either through soluble factors such as CXCL12, Kit ligand (also known as SCF), angiopoietin-1, and VCAM-1, or through contact-dependent signals.Modified from original figure design by Daniel Lucas, PhD, and Sandra Pinho, PhD, both from Albert Einstein College of Medicine.
Schematic Representation Illustrating the Major Cellular Constituents of the Bone Marrow HSC Niche. A variety of cells, including osteoblasts, Cxcl12-abundant reticular (CAR) cells, Nestin+ mesenchymal stem cells (MSC), Leptin receptor (Lepr)-expressing perivascular cells, and endothelial cells, have been reported as possible components of the niche. Schwann cells, wrapping sympathetic nerve fibers, promote HSC quiescence. In total, the structural makeup of these various cells provides a specialized microenvironment regulating HSC self-renewal and differentiation, either through soluble factors such as CXCL12, Kit ligand (also known as SCF), angiopoietin-1, and VCAM-1, or through contact-dependent signals.Modified from original figure design by Daniel Lucas, PhD, and Sandra Pinho, PhD, both from Albert Einstein College of Medicine.
That adult hematopoiesis takes place largely in the confines of bone cavities suggested that osteolineage cells might play an important role in HSC homeostasis.1 Several studies noted that progenitor activity was found under steady state, or after transplantation, near bone surfaces (Figure). The field took a giant leap forward following advances in mouse genetics and imaging capabilities. Studies using those techniques revealed both that alterations in osteoblast function could lead to significant changes in HSC numbers and that HSCs localized near osteoblasts in the endosteum (Figure). However, that there are many more osteoblasts than HSCs in the bone marrow begged the question, which osteoblast acts as the niche cell?
Sometimes scientific advances come serendipitously from unexpected directions. Searching for novel HSC markers, SLAM antigens (CD150 and CD48) were found to discriminate between HSCs and lineage-committed cells, and based on these phenotypic characteristics, most HSCs were found (using imaging analyses) to localize near blood sinusoids (Figure) rather than near osteoblasts.2 Other studies have found that β-adrenergic signals from the sympathetic nervous system (SNS) promote HSC mobilization from the bone marrow (Figure).3 These adrenergic signals were also found to be regulators of circadian HSC egress under homeostatic conditions, suggesting a role of SNS nerves in physiological regulation of the niche.4 Consistent with the perivascular location of HSCs, SNS nerves associate closely with pericyte-like cells identified through transgenic expression of the green fluorescent protein (GFP) under control of the Nestin gene promoter elements.5 These Nestin-GFP+ cells are physically associated with HSCs and express high levels of genes involved in HSC maintenance such as stem cell factor (Scf), Cxcl12, angiopoietin-1, and vascular cell adhesion molecule-1 (Vcam1) (Figure). After administration of granulocyte-colony stimulating factor or β3 adrenergic agonists, these four “retention” factors are downregulated selectively in Nestin-GFP+ cells, but not in other bone marrow stromal cells, indicating that these specialized cells have a functional role in the regulation of HSC behavior.
The niche itself is also regulated by hematopoietic cells. For example, regulatory T cells protect allogeneic HSCs in their niche, allowing their persistence for long periods in the bone marrow even when the recipient marrow was not treated with a preparative regimen.6 In addition, depletion of mononuclear phagocytes using clodronate liposomes increased the number of circulating HSCs.7-9 This effect was mediated by bone marrow CD169+ macrophages that secrete factor(s) that promote the synthesis of the four aforementioned HSC retention genes by Nestin-GFP+ cells(Figure).7
A prevalent idea in the field suggests that two niche compartments, the osteoblastic and vascular niches, may house distinct types of stem cells. For example, it has been suggested that the osteoblastic (also called “endosteal”) niche may promote HSC quiescence and that HSCs may then migrate to the vascular niche to proliferate and differentiate. While this idea may be intellectually appealing, supporting evidence remains controversial. Cell-cycle quiescence is a hallmark of stem cells, as it is thought to protect them from exogenous stress. Searching for sites enabling the activation of transforming growth factor-β (TGF-β), a powerful factor capable of inducing HSC quiescence, glial fibrillary acidic protein (GFAP)+ Schwann cells that ensheath SNS nerve fibers were reported to promote TGF-β activation.10 These GFAP+ cells, and the nerves that they protect, are found around blood vessels in the bone marrow, suggesting that HSC quiescence is promoted in the “vascular niche.” Although both the vascular niche and the putative osteoblastic HSC niche are broadly distributed in the bone marrow, differences in HSC proliferative and homing capacity have been reported between cells harvested from the endosteal or central regions.11 Whether identical perivascular structures comprise all HSC niches remains unclear at present. However, there is evidence that perivascular niches are subjected to different signals when situated near osteoblasts compared to when they are situated away from osteoblasts.
The complexity of the niche was further illustrated by a recent study in which the cell origin of stem cell factor (SCF) production, the ligand of c-kit receptor, was investigated. It has been known for many years that SCF is produced in non-transplantable bone marrow stromal cells. When expressed under the Scf locus, GFP expression was found to be localized primarily around sinusoids (Figure).12 Additional studies using transgenic models suggested that SCF was produced by both endothelial cells and a subset of perivascular cells (leptin receptor-positive, LepR+ cells) (Figure). It is important to note that although this study proposed that LepR+ cells are distinct from nestin-positive cells, nestin-positive cells were reported to express high levels of leptin receptor transcripts,5 suggesting some overlap between the two putative niche components.
The emerging contributions of multiple stromal cell types to the HSC niche raise the bar for translational medicine aimed at expanding HSC for transplantation. Although we know of a few secreted or contact factors that regulate HSC maintenance, most signals that HSCs integrate to make fate decisions remain unknown. If these signals were provided by different cells, one can imagine the difficulty of creating a multicellular niche that will meet the rigorous regulatory criteria for clinical cell therapy. Additionally, the propensity of HSCs to remain quiescent represents a significant challenge to bringing ex vivo expanded stem cells to the patient. The solution for clearing these hurdles will undoubtedly be found through greater understanding of the cellular and molecular basis of the niche. Hearteningly, the niche is evolving in our minds much faster than it does in nature. We should be catching up soon.
References
Competing Interests
Dr. Frenette indicated no relevant conflicts of interest.