Update/Commentary
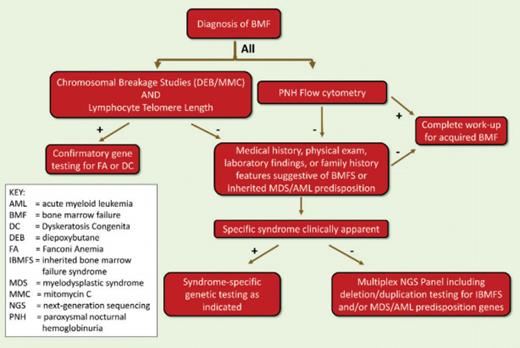
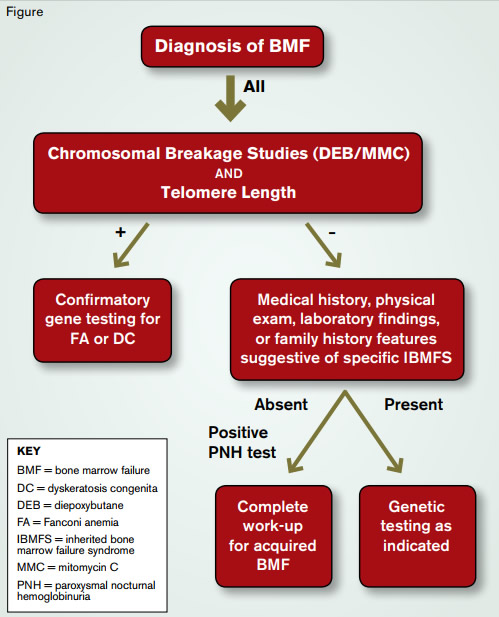
Figure 2. Evaluation of Bone Marrow Failure in Children and Young Adults
Figure 2. Evaluation of Bone Marrow Failure in Children and Young Adults
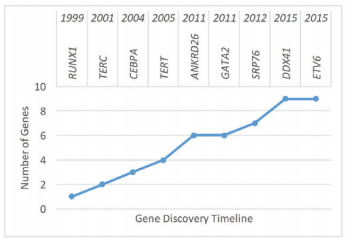
Figure 1. Timeline of Discovery of the Emerging Inherited Myelodysplastic Syndrome/Acute Myeloid Leukemia Predisposition Syndromes.
Figure 1. Timeline of Discovery of the Emerging Inherited Myelodysplastic Syndrome/Acute Myeloid Leukemia Predisposition Syndromes.
With the increasing use of next-generation sequencing (NGS), our understanding of the genetics underlying inherited bone marrow failure syndrome (IBMFS) and predisposition to myelodysplastic syndrome (MDS) has grown significantly. The numbers of genes linked to classic IBMF, such as Fanconi Anemia (FA; ≥ 17 genes), Diamond Blackfan Anemia (DBA; ≥ 14 genes), and Dyskeratosis Congenita (DC; ≥ 11), have increased. Additionally, throughout the last five years, five new genes (GATA2, ANKRD26, SRP76, DDX41, and ETV6) have been linked to familial MDS and leukemia (Figure 1). Importantly, it is increasingly recognized that these conditions can initially present in adulthood. With the improved knowledge of the inherited BMF and MDS, the differential diagnosis for a young adult presenting with BMF or myelodysplasia should be broadened to include not only the classical IBMFSs such as DC and FA, but also the emerging familial MDS/AML predisposition syndromes. Although the knowledge of these disorders remains imperfect, we expect that our diagnostic ability will improve as more genes and syndromes are identified. Presently, the comprehensive evaluation of a young adult with a suspected IBMFS or familial MDS/leukemia syndrome (Figure 2) continues to rely on functional testing of affected biological pathways to screen for the classic polygenic IBMFSs DC and FA. With the ready availability of clinically approved comprehensive NGS panels targeting genes known to cause IBMFS and familial MDS/AML, these panels have become a useful and frequently more economical alternative to sequential single-gene testing, particularly in cases in which syndromic features suggestive of a specific diagnosis are not present.
The Case
The patient is a 29-year-old male who presented with a two year history of progressive pancytopenia (WBC 2200/μL, ANC 830/μL; Hb 9.9g/dL with 2.9% reticulocytes; platelet count 26,000/μL). He was of short stature with discrete facial dysmorphisms and mildly impaired cognitively. The family history was negative for bone marrow failure (BMF), but there was an extensive history of early cancer on the maternal side. Bone marrow examination revealed marked hypocellularity without dysplasia or excess blasts. The patient became transfusion-dependent, so an unrelated bone marrow transplant (BMT) had been recommended as definitive treatment.
The Question
How do you evaluate a young adult with BMF? What features suggest an underlying inherited bone marrow failure syndrome (IBMFS) as the etiology? How do you evaluate for these disorders, and why is it important?
Our Response
IBMFS is a collection of genetic disorders including Fanconi anemia (FA), dyskeratosis congenita (DC), Diamond-Blackfan anemia (DBA), Shwachman-Diamond syndrome (SDS), severe congenital neutropenia, and congenital megakaryocytic thrombocytopenia that are associated with insufficient blood cell production, congenital anomalies, and, in some cases, an increased risk of malignancies including myelodysplastic syndrome (MDS), leukemia, and certain solid tumors.1 IBMFS is not always inherited. Disease may also occur sporadically due to a de novo mutation, which may account for more than 50 percent of all cases for some of the IBMFS (e.g., certain genetic forms of DBA or DC). Sporadic appearance of disease may also be due to low penetrance of the causative mutation or genetic anticipation in which clinical phenotype becomes more severe in successive generation as observed in autosomal dominant DC. In this case, family members may carry the mutation but not have clinical symptoms (silent mutation carriers). IBMFS can affect one, two, or all blood cell lineages. Abnormal blood cell counts are frequently not present at birth but develop during childhood, adolescence, or later in life.2 In some cases, BMF never becomes clinically manifest, and the affected individual may seek medical attention because of an unusually early onset of MDS, leukemia, cancer, or other non-hematologic manifestations associated with the condition, such as pulmonary fibrosis in individuals with DC.3 While once thought to be strictly the domain of pediatric hematologists, with improved insights into the spectrum of disease expression and genetic testing, IBMFS is increasingly being diagnosed and cared for in “adult” hematology clinics.4
When?
For all patients presenting with BMF, the possibility of an underlying IBMFS should be considered. Such consideration is particularly relevant for patients in whom BMT is being considered as treatment. IBMFS should also be considered in children and young adults presenting with MDS or MDS/leukemia, as these clonal disorders are late complications of IBMFS and may be the first symptom of disease in about 20 percent of cases, typically in the young adult population.3 IBMFS, with disease onset in adults, often lack the classic physical stigmata, such as abnormal thumbs (FA) or dystrophic fingernails (DC), and other extra-hematopoietic manifestations, such as small stature or discrete facial anomalies, may be subtle.4 Therefore, it is difficult to propose an age limit for the exclusion of an IBMFS, as these disorders may be diagnosed even in old age. However, the therapeutic consequences are probably most significant when diagnosed in pediatric and young adult patients. The diagram below suggests the diagnostic genetic workup for the young adult presenting with BMF.
What?
Currently, there are more than 80 different genes that may cause IBMFS.5 With the technology of whole-exome and whole-genome sequencing, the number of genetic abnormalities associated with IBMFS is expected to increase dramatically, making it impractical to perform testing for every gene in every affected individual. Many of the mutated gene products fall into distinct biologic pathways (e.g., the FA DNA repair pathway, the maintenance of telomeres in DC, or ribosome biogenesis in DBA). Some clinically available screening tests investigate the integrity of the affected pathway and, if abnormal, direct a focused and more economical approach to genetic testing. Given the important therapeutic implications associated with diagnosis and the availability of sensitive diagnostic tests, screening for FA and DC is recommended in every patient presenting with BMF or early MDS/leukemia. Due to abnormal DNA repair, chromosomes of dividing cells of patients with FA are hypersensitive to breakage induced by cross-linking agents such as diepoxybutane (DEB) or mitomycin C (MMC), and this characteristic is the basis of the diagnostic test for the disease.6 The DEB/MMC sensitivity test uses patient lymphocytes in the analysis and is available in several major medical centers with a specific interest in FA. Telomeres of nucleated peripheral blood cells are excessively short in DC.7
Clinical telomere length measurements by PCR, flow cytometry, or Southern blotting are available through several commercial laboratories. Results are usually provided as “percentile” in comparison to normal controls. The interpretation of the clinical significance of the measured telomere lengths can be difficult and often needs to be done in the context of disease manifestations. In general, telomere lengths within the normal distribution exclude DC to be the cause of BMF. In children and young adults with BMF caused by DC, the telomere lengths of total peripheral blood lymphocytes are usually 2 to 3 standard deviations below the telomere lengths distribution of age-matched healthy controls. Short telomere lengths in granulocytes are less specific for DC and can be short in a number of other IBMFS. As telomeres get shorter with age, telomere length becomes less diagnostic for DC in older individuals. If one of these screening tests is abnormal, subsequent genetic testing should be pursued, as only the identification of a disease-causing mutation can confirm the diagnosis of an IBMFS. More specific tests for other IBMFS have to be chosen on an individual basis depending on clinical presentation, family history, and laboratory findings. For example, DBA may be considered in patients who present with macrocytic anemia, low reticulocyte count, and a bone marrow characterized by an isolated erythroid hypoplasia. An elevated erythrocyte adenosine deaminase activity may further support a diagnosis of DBA. A history of symptoms of pancreatic insufficiency in a patient with earlyonset MDS may be suggestive for SDS. Genetic testing for mutations in the ribosomal proteins most frequently associated with DBA and for mutations in SBDS (the gene that is mutated in SDS) are available commercially. With wider applicability of novel sequencing technologies, genetic testing for all major IBMFS genes using a single multiplex platform is likely to become available in the not-too-distant future. However, with the increased availability of genetic testing, the interpretation of results and consideration of their clinical significance will become more complicated, requiring management by an experienced team of physicians that includes geneticists, hematopathologists, and hematologists.
Why?
The natural history, response to treatment, and surveillance requirements differ significantly between patients with acquired BMF and IBMFS. Patients with IBMFS are typically not responsive to immunosuppressive therapies that may be used for acquired aplastic anemia. Furthermore, the presence of IBMFS requires member donor selection and choice of transplant conditioning regimen, as conventional conditioning regimens can be excessively toxic or fatal for some of the IBMFS.8 IBMFS may require surveillance for long-term complications that may be accelerated by the choice of treatment (pulmonary fibrosis, osteopenia, MDS/leukemia, and other non-hematologic malignancies). Finally, the diagnosis of IBMFS requires informed genetic counseling for both the affected individual and the family.
In the patient presented above, testing of peripheral blood lymphocytes revealed telomere lengths within the normal distribution, excluding DC as the cause of his progressive BMF.9 No increased chromosomal sensitivity to DEB was observed in his peripheral blood lymphocytes and only a mildly increased chromosomal fragility with the formation of few radials using MMC was observed. These findings were not sufficient for a firm diagnosis but raised our suspicion of FA and led to further testing of skin fibroblasts that demonstrated significant chromosomal breakage and numerous radial formations with both DEB and MMC. Together, these results suggested FA as the cause of the patient’s progressive BMF. Genetic reversion (the process by which a lymphohematopoietic cell undergoes spontaneous correction of an FA-causing gene mutation) and blood mosaicism are known to occur in about 15 percent of FA patients.10 This process is the likely cause of the ambiguous FA screening results from the peripheral blood lymphocytes in this patient.
References
Author notes
The update/commentary section was added in 2016 when this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium.
Competing Interests
The authors indicated no relevant conflicts of interest.