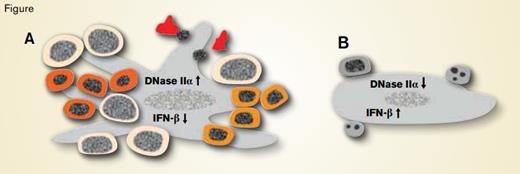
Erythroblastic Islands of Fetal Liver of A) Wild-Type and B) EKLF/KLF1 Null Mice. Central macrophages, large gray cells with elongated ovoid nuclei, have cytoplasmic extensions in wild-type but not EKLF/KLF1 null fetal livers. In wild-type central macrophages, EKLF/KLF1 induces DNase IIα, which degrades DNA of phagocytosed apoptotic cells and extruded erythroid nuclei, thereby inhibiting interferon-β (IFN-β) production. In EKLF/KLF1 null central macrophages, EKLF/KLF1 deficiency decreases DNase IIα, which increases undigested DNA, inducing IFN-β production. In wild-type mice, EKLF/KLF1 regulates many genes involved in terminal erythroblast (round nucleated cells) differentiation, including those related to cell division. Consequently, cell size decreases, hemoglobin accumulates (shown as increasingly orange-red cytoplasm), and nuclear condensation and extrusion occur (shown with the extruded nucleus nearby the irregularly shaped, hemoglobin-filled reticulocytes (A). In EKLF/KLF1 null erythroblasts (B), defective terminal differentiation (shown by no orange-red in cytoplasm) due to deficient EKLF/KLF1 combined with increased IFN-β from the central macrophage leads to extensive erythroblast apoptosis (fragmented nuclei) and failure of definitive erythropoiesis.
Erythroblastic Islands of Fetal Liver of A) Wild-Type and B) EKLF/KLF1 Null Mice. Central macrophages, large gray cells with elongated ovoid nuclei, have cytoplasmic extensions in wild-type but not EKLF/KLF1 null fetal livers. In wild-type central macrophages, EKLF/KLF1 induces DNase IIα, which degrades DNA of phagocytosed apoptotic cells and extruded erythroid nuclei, thereby inhibiting interferon-β (IFN-β) production. In EKLF/KLF1 null central macrophages, EKLF/KLF1 deficiency decreases DNase IIα, which increases undigested DNA, inducing IFN-β production. In wild-type mice, EKLF/KLF1 regulates many genes involved in terminal erythroblast (round nucleated cells) differentiation, including those related to cell division. Consequently, cell size decreases, hemoglobin accumulates (shown as increasingly orange-red cytoplasm), and nuclear condensation and extrusion occur (shown with the extruded nucleus nearby the irregularly shaped, hemoglobin-filled reticulocytes (A). In EKLF/KLF1 null erythroblasts (B), defective terminal differentiation (shown by no orange-red in cytoplasm) due to deficient EKLF/KLF1 combined with increased IFN-β from the central macrophage leads to extensive erythroblast apoptosis (fragmented nuclei) and failure of definitive erythropoiesis.
Among vertebrates, mammals and birds have small erythrocytes with high surface-to-volume ratios that optimize oxygen delivery for the high-energy requirements of warm-blooded animals. With relatively smaller genome sizes, birds can meet the high surface-to-volume ratio requirement by producing small erythrocytes with tightly condensed nuclei, while mammals with their larger genomes produce small erythrocytes by extruding the nucleus at the orthochromatic erythroblastic stage of maturation.1 Newly produced mammalian erythrocytes circulate for months, but the extruded nuclei are rapidly phagocytosed with subsequent DNA degradation and recycling of nuclear constituents. Nuclear disposal is mediated by central macrophages located in erythroblastic islands. The erythroblastic island is the functional unit of definitive mammalian erythropoiesis and consists of a central macrophage surrounded by adherent erythroblasts at various stages of differentiation (Figure). Rigorous studies by Porcu et al. demonstrate that erythroid Krüppel-like factor (EKLF/KLF1), a zinc-finger transcription factor that binds CACC sequences in regulatory regions of genes expressed in differentiating erythroblasts, controls expression of DNase IIα in central macrophages of erythroblastic islands. Because definitive mammalian erythropoiesis requires DNase IIα for erythroid nuclear disposal in central macrophages, EKLF/KLF1 plays a key role in coordinating the function of both macrophages and erythroid cells in erythroblastic islands.
Extensively characterized in relation to β-globin transcription, EKLF/KLF1 also regulates genes involved in the erythroblast cell cycle and in erythrocyte membrane and cytoskeletal development.2 Human erythroid diseases with EKLF/KLF1 mutations include hereditary persistence of fetal hemoglobin, Lutheran antigen deficiency, and congenital dyserythropoietic anemia. EKLF/KLF1 knockout mice have lethal anemia as a result of failure of definitive erythropoiesis in the fetal liver. Although the α-globin/β-globin ratio in erythroid cells of EKLF/KLF1 null embryos could be improved experimentally using a transgene expression approach, the animals still died, indicating that EKLF/KLF1-targeted, non-globin genes contribute to the embryonic-lethal anemia.
That EKLF/KLF1 deficiency in non-erythroid cells might contribute to the embryonic-lethal anemia was suggested by DNase IIα knockout mice. DNase IIα, a lysosomal endonuclease, digests DNA of phagocytosed apoptotic cells and, in erythroblastic island central macrophages, DNA of phagocytosed erythroblast nuclei.3 Similar to EKLF/KLF1 null mice, DNase IIα knockout mice have a lethal anemia as a consequence of failure of fetal liver definitive erythropoiesis. Central macrophages of erythroblastic islands in DNase IIα null mice accumulate undigested DNA that triggers macrophage release of interferon-β that induces apoptosis of the surrounding erythroblasts.
In Brief
Porcu et al. demonstrate that EKLF/KLF1 binds the promoter region and up-regulates DNase IIα gene transcription in murine fetal liver macrophages. In EKLF/KLF1 null mice, fetal liver DNase IIα content and activity were decreased while interferon-β expression was increased, conditions similar to those observed for DNase IIα null mice. EKLF/KLF1 null fetal livers contained fewer macrophages, and the macrophages that were present had decreased cytoplasmic extensions (Figure), suggesting other non-erythroid effects of EKLF/KLF1 deficiency. Intrinsic defects during terminal differentiation of EKLF/KLF1 null erythroblasts likely increase apoptosis, and the DNase IIα-deficient central macrophages are unable to accommodate the resulting apoptotic burden in addition to erythroid nuclear disposal. These results reinforce the notion
References
Competing Interests
Dr. Koury indicated no relevant conflicts of interest.