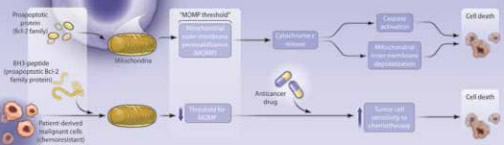
"Adjustment of the Threshold for Apoptosis. Top: Sensitive cells have mitochondria with a high pro- to anti-apoptotic Bcl-2 family protein ratio.
"Adjustment of the Threshold for Apoptosis. Top: Sensitive cells have mitochondria with a high pro- to anti-apoptotic Bcl-2 family protein ratio.
The characteristics that trigger differences in chemosensitivity between different tumor types and different individual malignancies remain largely unexplained. It is not clear why some neoplasms are sensitive to a range of agents with very different mechanisms of action, while others remain broadly resistant. Apoptosis induced by loss of mitochondrial integrity is an important pathway of chemotherapy-induced cell death, and one effect of mitochondrial outer membrane permeabalization (MOMP) in response to cytotoxic therapy is the release of cytochrome c, which, in turn, activates the caspase cascade in the cytosol, leading to programmed cell death (Figure). Control of MOMP is effected by members of the Bcl-2 protein family, and some years ago, overexpression of Bcl-2 was reported to render cells much less sensitive to cytotoxic drugs in vitro while clinically, chemotherapy failure was observed to correlate with Bcl-2 expression.1 Subsequent studies investigated the roles of other Bcl-2 family proteins, some of which are anti-apoptotic (Bcl-2, Mcl-1) and some pro-apoptotic (BAX, BAD). A crucial feature of this family is their capacity to oligomerize, with the balance of pro- and anti-apoptotic partners determining the effect on MOMP. A key region of interaction, the BH3 domain, can be bound by a variety of smaller BH3-only proteins (BIM, NOXA, BMF, PUMA) that exert a powerful pro-apoptotic effect.
This study from the Department of Medical Oncology at the Dana-Farber Cancer Institute sheds light on one possible determinant of chemosensitivity. Using a small library of different peptides from pro-apoptotic BH3-only proteins to induce MOMP, the investigators devised an assay to determine the intrinsic “fragility” of the mitochondria in different cell types. The MOMP effect of these peptides was assessed using a fluorescent dye, JC-1, which measures the inner mitochondrial membrane potential and can be detected using flow cytometry. What they found was that in a variety of hematologic malignancies (e.g., AML, ALL, myeloma) the degree of mitochondrial depolarization on exposure to the BH3-peptide library in tumor material ex vivo was a predictor of clinical responses to chemotherapy, whether reflected by the decline in paraprotein level for myeloma, the probability of remission induction in AML, or the durability of remission in ALL. There was also a correlation between BH3 peptide-induced mitochondrial fragility and both CA-125 response and progression-free survival in ovarian cancer. Which BH3 peptide was the best predictor varied between tumor types. For myeloma, it was BMF; for AML, it was BIM; and for ALL or ovarian cancer, it was PUMA. Having observed these correlations, the investigators suggested that mitochondrial priming to apoptosis has therapeutic potential, by showing that the BH3 mimetic drug ABT-737 increased chemosensitivity in the CML K562 cell line. Finally, they examined the sensitivity of mitochondria from a variety of normal tissues to priming and found that those that are considered chemo-resistant, such as the kidney, ovary, myometrium, and foreskin showed much lower sensitivity to BH3 peptides than malignant cells.
In Brief
The search for a reliable in vivo chemosensitivity test has been going on for many years, and, to date, none have proven convincing or readily applicable in the clinic. Although the number of cases studied here is relatively small, the preliminary data are encouraging and merit further exploration in prospective clinical trials. The idea that targeting the mitochondria with BH3 mimetics in chemoresistant tumors may improve results is especially appealing, and this assay may provide a method to identify those cases in which it might be most successful.
References
Competing Interests
Dr. Johnson indicated no relevant conflicts of interest.