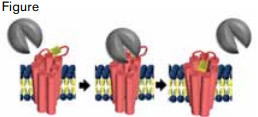
Thrombin (gray) Associates With PAR1 (pink) and Cleaves the N-Terminus, Revealing a Tethered Ligand (yellow).
Thrombin (gray) Associates With PAR1 (pink) and Cleaves the N-Terminus, Revealing a Tethered Ligand (yellow).
Protease-activated receptor 1 (PAR1) is a critical platelet receptor linking the coagulation cascade to activation of platelets during thrombosis. It is an attractive target for an antithrombotic because of its established importance in arterial clotting. Over the past decade, several programs to develop PAR1 antagonists have been launched. However, these programs have encountered multiple complications peculiar to this unusual receptor. PAR1 is activated by a tethered ligand generated by cleavage of the N-terminus by thrombin (Figure). To prevent this tethered ligand from interacting with its binding site, an active site inhibitor must have special pharmacologic properties such as tight binding and a slow off-rate. Animal testing of PAR1 inhibitors is complicated by the fact that most species use PAR4 rather than PAR1 to activate platelets. For this reason, the usual rodent repertoire used in preclinical studies for drug development cannot be relied on for development of drugs that inhibit PAR1. Clinical trials of PAR1 antagonists must consider the bleeding risk associated with anti-platelet drugs. Despite these challenges, vorapaxar (formerly SCH 530348) has emerged as the preeminent PAR1 inhibitor. In the highly anticipated phase III clinical trial, TRACER, Tricoci et al. have now evaluated vorapaxar in a study large enough to critically assess the efficacy and safety of this new antithrombotic strategy.
TRACER is a double-blind, randomized trial that enrolled 12,944 patients with acute coronary syndromes. Vorapaxar was compared with placebo in patients receiving standard antiplatelet regimens, including aspirin, clopidogrel, or both. The primary endpoint was composite death from cardiovascular causes, myocardial infarction, stroke, rehospitalization secondary to recurrent ischemia, or urgent coronary revascularization. After a median of 502 days of followup, 18.5 percent of patients receiving vorapaxar and 19.9 percent of patients receiving placebo had reached the primary endpoint (hazard ratio of 0.92, p=0.07). Death from cardiovascular causes was decreased with vorapaxar use (hazard ratio of 0.89, p=0.02). However, since superiority with respect to the primary endpoint was not achieved, superiority with respect to this secondary endpoint could not be declared. The safety results were more sobering. Rates of moderate to severe bleeding were significantly increased (hazard ratio of 1.35, p<0.001) and rates of intracranial hemorrhage were markedly increased (hazard ratio of 3.39, p<0.001). Overall, these results indicate a non-significant trend toward improved efficacy at the cost of a significant increase in bleeding. The increased bleeding risk in patients receiving vorapaxar led to the early termination of the study in patients with a history of stroke.
In Brief
Recurrent myocardial infarction or stroke despite current antithrombotic therapy is common and associated with a high mortality rate. There is a clear and pressing need to improve prevention in this setting. Phase II studies using vorapaxar in TRA-PCI raised the possibility that inhibition of PAR1 could be added to inhibition of thromboxane A2-mediated pathways (by aspirin) and P2Y12-mediated signaling (by clopidogrel) without increasing bleeding risk.1 That this result was not borne out in the TRACER trial has forced a re-evaluation of what role PAR1 antagonism might play in antithrombotic therapy. Recently, Merck announced that in a second large phase III trial, TRA-2P, vorapaxar significantly reduced the primary endpoint of cardiovascular death, heart attack, stroke, or urgent revascularization, but caused increased bleeding, including intracranial hemorrhage.2
The results of these phase III trials raise several questions regarding the path forward for PAR1 antagonists. Is there a way to identify susceptible patients so as to avoid intracranial hemorrhage? Should PAR1 antagonism be tested as replacement for, rather than supplementation of, clopidogrel, aspirin, or both? Could the risk-versus-benefit ratio of PAR1 antagonism be improved by modifying the duration of vorapaxar therapy? Might PAR1 inhibitors with different pharmacologic properties demonstrate an improved risk-versus-benefit ratio compared with vorapaxar? Although we have learned much about PAR1, the recent phase III clinical experience with vorapaxar indicates that we have more to learn about this fascinating receptor before we can apply this knowledge in the clinic.
References
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.