Fetal hemoglobin (HbF, α2γ2) is the main oxygen transport protein in the fetus during the last months of embryonic development and during the first few months of life after birth. The β-chain of adult hemoglobin (HbA, α2β2) and the γ-chain of HbF are encoded by a cluster of genes called the β-globin locus. In a complex, highly regulated process called hemoglobin switching, HbF is nearly completely replaced by HbA at six months of age.
Sickle cell disease (SCD) is caused by substitution of valine for glutamic acid at amino acid 6 in the β-globin chain of HbA, which produces HbS (α2βS2). Deoxygenation of HbS results in its polymerization, which starts a chain of events that includes red cell sickling, hemolysis, microvascular occlusion, and painful crises. HbF inhibits sickling by interfering with the polymerization of hemoglobin S. Thus, the switch from HbF to HbA is critical to the pathogenesis of SCD and the β-thalassemias. Hydroxyurea and other pharmacologic agents have been identified that promote the production of HbF, and hydroxyurea has been shown in randomized clinical trials to decrease the frequency of painful crises in human SCD.1
Recent human genetic studies have identified the gene encoding BCL11A as a locus that is important for control of HbF synthesis.2 Subsequent studies in mice and in human erythroid cell culture have demonstrated that BCL11A is a transcriptional factor that represses HbF synthesis.3 Knockout of the BCL11A gene in mice is postnatally lethal. To circumvent this difficulty, Xu et al. in the laboratory of Stuart Orkin at Harvard Medical School examined the contribution of BCL11A to HbF synthesis in non-SCD adult transgenic mice carrying the human β-globin gene cluster on a yeast artificial chromosome transgene and in an SCD mouse model.
HbF constituted greater than 80 percent of the hemoglobin population in fetal liver in mice manipulated to have erythroid-specific inactivation of BCL11A, and these animals developed normally and displayed normal erythropoiesis in fetal liver and adult bone marrow. After birth, the level of γ-globin declined to a level of ~11 percent in adults, indicting the presence of other, yet unidentified regulators of HbF synthesis. The expression of erythroid transcriptional regulators, including GATA1, FOG1, NF-E2, KLF1, SOX6, and MYB, in adult bone marrow of BCL11A-null erythroid cells was normal. These results demonstrated that BCL11A is highly selective and appears to control only the expression of globin genes.
To determine whether the γ-globin gene that is silenced during postnatal development can be reactivated, the BCL11A gene was deleted in the human β-globin transgenic adult mice. This was accomplished using a “floxed” BCL11A gene, which allowed it to be excised after activation of Cre recombinase, which in turn was under control of an interferon-inducible promoter. Inactivation of BCL11A resulted in a sustained increase of γ-globin synthesis to 14 percent of total β-like human globins. Additionally, BCL11A enhanced effects of the known HbF inducers, 5-aza-2’-deoxycytidine and suberoylanilide hydroxamic acid.
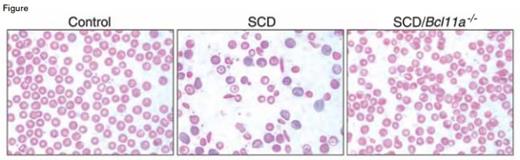
Sickled Red Cells Were Absent in SCD/Bcl11a-/- Mice. From Xu et al. Science 334:6058 (18 Nov 2011). Reprinted with permission from AAAS.
Sickled Red Cells Were Absent in SCD/Bcl11a-/- Mice. From Xu et al. Science 334:6058 (18 Nov 2011). Reprinted with permission from AAAS.
Next, Xu et al. determined whether inactivation of the BCL11A gene could be used to ameliorate disease symptoms in the Berkeley mouse model of SCD. These mice produce human HbS and low levels of HbF and develop many of the pathologic features of human SCD. BLC11A-floxed SCD mice, called SCD/Bcl11a-/- mice, displayed a marked increase in expression of HbF. Red blood cells containing HbF, called HbF cells, were also markedly elevated in SCD/Bcl11a-/- compared with control non-SCD and SCD mice. The level of HbF expression achieved by reducing BCL11A expression exceeded levels estimated to be necessary to eliminate the pathologic features of SCD. Abnormalities in red cell counts, red cell hemoglobin content, red cell survival, reticulocyte counts, and white blood cell counts were corrected in the SCD/Bcl11a-/- mice. Sickled red cells were absent in SCD/Bcl11a-/- mice (Figure), and splenomegaly was markedly decreased. Additionally, urine osmolality, which is abnormal in human and murine SCD, was normal in SCD/Bcl11a-/- mice.
In Brief
The study identifies BCL11A as a pharmacologic target for treatment of patients with SCD. The authors propose that this approach also may apply to the β-thalassemias. BCL11A is a transcription factor, which makes it a difficult target. However, the potential treatment for SCD by interfering with the function of a single component involved in globin gene regulation will likely spur extensive efforts to overcome this barrier.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.