Cloning of the human gene that encodes erythropoietin (EPO) allowed pharmacologic production of the principal hormonal regulator of erythropoiesis. Recombinant EPO has prevented transfusions and improved the life of many patients with renal failure-related and other hypoproliferative anemias. However, lack of knowledge about EPO gene regulation in the kidney, the major organ of production, has resulted in problems such as worsening of hypertension and lifethreatening thromboses in EPO-treated patients. EPO-producing cells were identified by in situ hybridization as a subset of fibroblasts located in the renal cortical interstitium adjacent to proximal tubules, an area sensitive to fine gradations of hypoxia due to a defined blood supply and high oxygen consumption rates. While they supply about 90 percent of the hormone in post-natal life, a purified population of these interstitial EPO-producing cells with which to study gene regulation had been unavailable until Pan and colleagues developed congenitally anemic mice with renal Epo-producing cells that express green fluorescent protein (GFP) (Figure).
Fetal liver is the major prenatal source of EPO, and hepatocytes produce the 10 percent of EPO not produced by the kidneys in post-natal life. Most molecular studies of EPO gene regulation utilized human hepatoma cell lines because they provided renewable, homogeneous populations of cells that produced EPO when rendered hypoxic. Using these hepatoma cells, the hypoxia-responsive elements (HREs) in the regulatory, non-coding regions of EPO were identified, including the extensively studied 3'-HRE enhancer. Subsequently, HREs have been found in numerous hypoxia-responsive genes involved in many aspects of cell biology including angiogenesis, glucose metabolism, and proliferation. Hypoxia-inducible factor1-α (HIF1α), a component of the transcription factor complex that binds HREs and up-regulates EPO transcription, was first described in hepatoma cell lines. Other discoveries with cell lines that have defined the mechanism by which HIF1α is regulated include prolyl hydroxylases that use molecular oxygen to hydroxylate HIF1α, recognition and ubiquitination of hydroxylated HIF1α by Von Hippel-Lindau protein, and rapid proteasomal degradation of hydroxylated HIF1α.
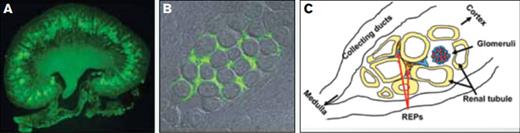
(A) Low power and (B) higher power photomicrographs of green fluorescence of renal erythropoietin-producing (REP) cells in the peritubular interstitial space of the inner cortex. (C) Diagram of REPs location. Modified from Pan X, et al. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One. 2011;6:e25839.
(A) Low power and (B) higher power photomicrographs of green fluorescence of renal erythropoietin-producing (REP) cells in the peritubular interstitial space of the inner cortex. (C) Diagram of REPs location. Modified from Pan X, et al. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PLoS One. 2011;6:e25839.
In Brief
Although HIF1α and 3'-HRE are important for hepatic EPO regulation, Hif2α regulates renal Epo transcription, and the 3'-HRE of Epo is not required for renal Epo production. Pan and colleagues created compound heterozygous mice in which one Epo allele was rendered nonfunctional by knock-in of a GFP cassette, while the other Epo allele had its 3'-HRE deleted. With the 3'-HRE deleted in the functional Epo allele, fetal liver Epo production was deficient in late gestation, and newborn mice were severely anemic with renal Epo-producing cells uniformly expressing GFP. Although renal Epo-producing cells represent 0.2 percent of total kidney cells, the loose structure of newborn kidneys and fluorescence-activated sorting of GFP-labeled, Epo-producing cells permitted gleaning of populations with greater than 75 percent purity. With these highly enriched renal Epo-producing cells, Pan and colleagues demonstrated that mRNAs for Hif2α and a splice variant of Hif3α, a negative regulator of the Hif pathway, are induced by hypoxia, while Hif1α mRNA is not. These results indicate that the renal cells responsible for the large majority of circulating Epo have a complex regulation of Epo transcription by Hif2α and Hif3α at HREs other than the canonical 3'-HRE. The purified population of renal Epoproducing cells should provide a means to understand how hypoxia regulates production of the large majority of EPO in human circulation and how EPO administration can be modified to relieve anemia while avoiding hypertensive and thrombotic complications.
Competing Interests
Dr. Koury indicated no relevant conflicts of interest.