Dr. Gotlib receives clinical trial funding from Incyte, Inc., Sanofi-Aventis, and YM Biosciences. He also serves on an advisory board and receives honoraria from Incyte, Inc.
Much has been learned after five years of phase I-II clinical trial experience with several JAK2 inhibitors in treatment of myelofibrosis, as well as the two phase III COMFORT trials that led to approval of ruxolitinib in 2011.1,2 Some tyrosine kinase inhibitors (TKIs) in this drug class are relatively more JAK2-specific (e.g., SAR302503, formerly TG101348),3 whereas others demonstrate inhibitory activity against both JAK1 and JAK2 (e.g., ruxolitinib and CYT387).1,2,4 Inhibition of JAK2 blocks JAK–STAT-mediated clonal proliferation of myeloproliferative neoplasm (MPN) cells, whereas inhibition of both JAK1 and JAK2 is felt to be responsible for interrupting the aberrant cytokine signaling that contributes to disease features such as hypercatabolic constitutional symptoms, ineffective hematopoiesis, and bone marrow fibrosis. JAK inhibitors have produced substantial clinical improvement on two fronts: reduction of splenomegaly and amelioration of debilitating constitutional symptoms. However, on-target, antiproliferative effects may mitigate these benefits and sometimes limit dosing of these agents in certain individuals by worsening anemia and thrombocytopenia. With only modest or no effects on JAK2 V617F allele burden and bone marrow fibrosis, and uncertain impact on long-term survival, today’s JAK inhibitors do not share the exceptionalism of imatinib and the next generation TKIs used to treat CML. The genetic complexity of MPNs and the lack of specificity of current JAK inhibitors for mutant JAK2 account for some of these differences in efficacy.
Disease persistence on JAK2 inhibitor therapy, or what I refer to as “disease creep,” is not an uncommon circumstance, and it usually manifests over time as a gradual return of splenomegaly and/or constitutional symptoms (in contrast to the more rapid return of symptoms within approximately one week after drug hold/discontinuation). The lack of indepth clinicopathologic responses with JAK2 inhibitors is also synonymous with the concept of disease persistence. Dr. Koppikar and colleagues from Dr. Ross Levine’s laboratory at Memorial Sloan-Kettering Cancer Center convincingly demonstrate that one mechanism of disease persistence relates to reactivation of JAK2 via transphosphorylation by JAK family members JAK1 and TYK2.
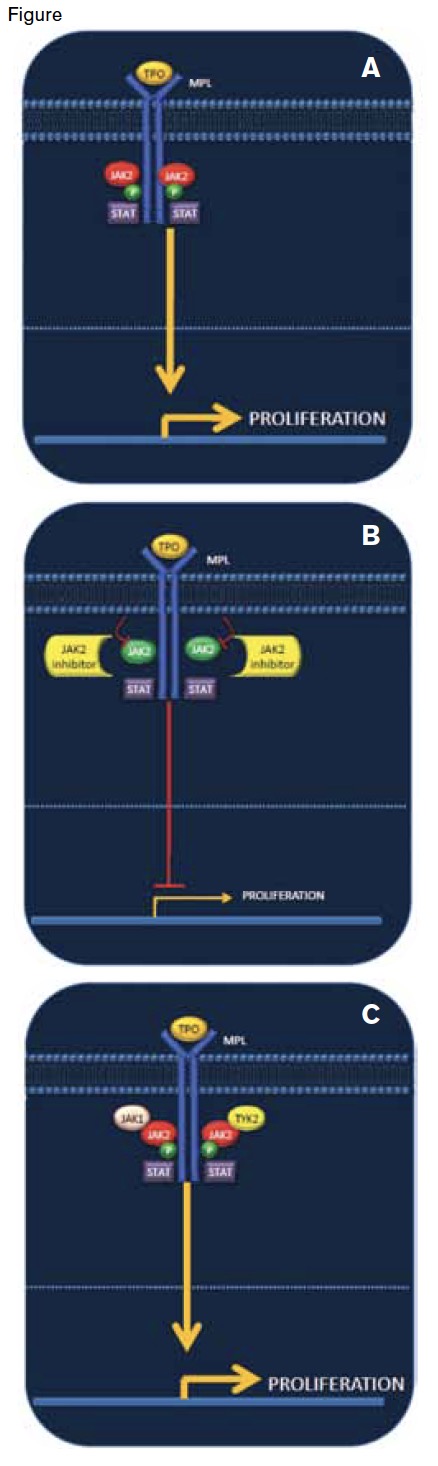
Binding of thrombopoietin (TPO) to its receptor (MPL) leads to phosphorylation of JAK2, activation of the JAK-STAT pathway, and increased cellular proliferation (A). JAK2 inhibitors block phosphorylation of JAK2 and reduce cellular proliferation and well as other signaling cascades (B). One mechanism of disease persistence in the setting of JAK2 inhibitor therapy is re-phosphorylation of JAK2 through its heterodimerization with JAK family members JAK1 and TYK2. The result is re-activation of the JAK–STAT pathway and downstream signaling events, including cellular proliferation (C).
Binding of thrombopoietin (TPO) to its receptor (MPL) leads to phosphorylation of JAK2, activation of the JAK-STAT pathway, and increased cellular proliferation (A). JAK2 inhibitors block phosphorylation of JAK2 and reduce cellular proliferation and well as other signaling cascades (B). One mechanism of disease persistence in the setting of JAK2 inhibitor therapy is re-phosphorylation of JAK2 through its heterodimerization with JAK family members JAK1 and TYK2. The result is re-activation of the JAK–STAT pathway and downstream signaling events, including cellular proliferation (C).
To unravel the biology of disease persistence, investigators first exposed different JAK2 V617F-positive cell lines to ruxolitinib or a pre-clinical pan-JAK inhibitor for several weeks and found that chronic exposure to these drugs resulted in continued proliferation and resistance to apoptosis at concentrations that prevented growth of the parental cell line. Cells were also resistant to other JAK inhibitors (e.g., SAR302503) to which they hadn’t been exposed. Sequencing of the JAK2 gene in persistent cells revealed no acquired resistance mutations akin to the BCR-ABL resistance mutations observed with TKIs in CML. In contrast to the observed inhibition of downstream signaling in untreated cells or treatment-naive MPN patient samples exposed to JAK2 inhibitors, persistent cell lines and granulocytes from patients on chronic ruxolitinib therapy displayed sustained phosphorylation of JAK2 and signaling intermediates, such as STAT3, STAT5, and MAP kinase. A consistent finding in the persistent versus treatment-naive cells or patient samples was the increased association between phosphorylated JAK2 and JAK family members JAK1 and TYK2. In addition, increased JAK2 mRNA expression and increased stability of the JAK2 protein was found to contribute to persistence and to facilitate heterodimerization formation between JAK family members. Withdrawal of JAK2 inhibitor treatment resulted in re-sensitization to different JAK inhibitors and loss of association of JAK2 with JAK1/TYK2. Knockdown of JAK1 and TYK2 reverted cells from a persistent to sensitive phenotype. An Hsp90 inhibitor that degrades JAK2 and a type II JAK inhibitor that also binds inactive JAK2 were found to retain the capacity to inhibit persistent cells to the same degree as naive cells, suggesting that alternative treatment approaches may be able to bypass the persistence phenomenon.
In Brief
These data highlight that reactivation of JAK2 via heterodimerization with JAK family members is a fundamental basis of MPN disease persistence in patients on JAK inhibitor therapy. Syncopated schedules of JAK inhibitor therapy with the intent of re-sensitizing patients to drug is unlikely to work given the rapid return of signs and symptoms of disease when treatment is discontinued. If we play our cards right, use of novel JAK inhibitors or their combination with therapeutics with non-overlapping mechanisms of action (e.g., inhibitors of PI3 kinase, Hsp90, histone deacetylase) may prove to be the winning hand.