Abstract
Regulatory T cells (TRs) can suppress the function of other effector T cells in the setting of autoimmunity, transplantation, and resistance to tumors. The mechanism for the induction of TRs has not been defined. We previously reported that an injection of immature dendritic cells (DCs) pulsed with influenza matrix peptide (MP) led 7 days later to antigen-specific silencing of effector T-cell function in the blood of 2 healthy human subjects. Here, we found that interferon-γ–producing effectors return by 6 months. Importantly, in mixing experiments, CD8+ T cells from the sample obtained 7 days after injection could suppress MP-specific effectors obtained before injection and those in recovery samples. This suppression or regulation was specific for the immunizing peptide (MP) and cell-dose dependent, and it required contact between the 2 samples. These data show the capacity of immature DCs to induce antigen-specific regulatory CD8+ T cells in humans.
Introduction
Regulatory T cells (TRs) have now been clearly identified in mice and humans.1-3 These cells can inhibit strong responses mediated by CD4+ and CD8+ effector T cells, thus preventing allograft rejection, graft-versus-host disease (GVHD), chronic inflammatory disease, and autoimmunity (for a review, see Roncarolo and Levings,1Waldmann and Cobbold,2 and Sakaguchi3). Recent studies have identified TRs in human blood, where they have 2 main functional properties4-6: they proliferate poorly in response to mitogenic stimuli and they can dampen the responses of effector T cells.7 Although most studies have characterized CD4+ TRs,8,9CD8+ T cells with regulatory properties have also been described.10-16
Certain populations of TRs, particularly those expressing CD4 and the CD25 interleukin 2 (IL-2)–receptor chain, are generated in the thymus, where the cortical epithelium has been identified as a critical antigen-presenting cell (APC).17TRs, often identified by their capacity to produce IL-10, can also be induced peripherally in the settings of transplantation and GVHD,1 2 but the APC requirements have not been identified. It is important to identify pathways that control the formation of TRs, since these would provide novel strategies for antigen-specific immune suppression or immune tolerance.
Dendritic cells (DCs) are powerful APCs for the induction of effector T cells.18 To initiate immunity, DCs must carry out 2 sets of linked events.19 One is the capture of antigens and successful formation of major histocompatibility complex (MHC)–peptide complexes; the second is to undergo a process termed “maturation” to acquire many additional properties to stimulate immunity.19 Immature DCs appear to be inactive as inducers of immunity in vivo.20 However, in a standard tissue-culture assay involving initiation of the mixed leukocyte reaction, immature DCs were not inactive but instead induced the formation of TRs, with both the anergic and regulatory properties mentioned above.21
In parallel, we tested the effects of immunizing volunteers with immature DCs. We reported findings in 2 healthy volunteers who received a single subcutaneous injection of 2 × 106 immature DCs pulsed with an HLA-A*0201–restricted influenza matrix peptide (MP).22 In contrast to previous findings using mature DCs,23 injection of immature DCs was associated with antigen-specific inhibition of effector T-cell function. The peptide-specific, interferon-γ (IFN-γ)–producing cells disappeared from the blood, and cytolytic cells could no longer be expanded in culture. However, antigen-binding CD8+ T cells were still present, and circulating MP-specific IL-10 producers developed. In summary, the use of immature DCs silenced effector T-cell functions, raising the possibility that TRs were being induced in vivo.
In the study described here, we showed that the loss of effector function and induction of IL-10 producers is self-limited, since there was a return to preimmunization status by 6 months after immunization. Importantly, we found that 1 week after immunization, the blood did contain CD8+ T cells with TR function, able to block the function of CD8+ effectors that secrete IFN-γ. These data provide evidence that immature DCs can induce antigen-specific CD8+ TRs in vivo in humans.
Materials and methods
Study design and injection of DCs
This report describes the findings in 2 volunteer subjects (Im1 and Im2) who received subcutaneous injections of immature DCs derived by culture of blood monocyte precursors in granulocyte-macrophage colony-stimulating factor and IL-4 as described previously.22 The injected DCs were pulsed with keyhole-limpet hemocyanin and influenza MP during the last 16 hours of a 6-day (Im1) or 7-day (Im2) monocyte culture as described previously.22 Approval was obtained from the institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki.
Follow-up and immune monitoring
Both subjects were evaluated 1 week after DC injection and at 1- to 3-month intervals afterward. Both had normal results on repeated hemogram assessments and no rheumatoid factor or antinuclear antibody 1 and 3 months after DC injection. Antigen-specific T cells were quantified by using a standard enzyme-linked immunospot (ELISPOT) assay for the presence of peptide-specific cells producing IFN-γ, IL-4, or IL-10.22 For cytolytic T-lymphocyte (CTL) assays, T cells were cocultured with peptide-pulsed mature DCs for a week, before measurement of lytic activity, as described previously.22DC maturation was achieved by day 1 of culture in a mixture of IL-1β, IL-6, tumor necrosis factor α, and prostaglandin E2.
Assays for TRs
Peripheral blood mononuclear cells (PBMCs) obtained 7 days after immunization (TR sample), before immunization, or at recovery (eg, day 180) were thawed and cultured (2-3 × 105 cells/well) either separately or together in the presence of peptide-pulsed, autologous monocyte-derived mature DCs at a PBMC-to-DC ratio of 60:1. Antigen-specific INF-γ–producing cells were quantified by using a standard ELISPOT assay as described previously.23 In addition to the immunizing peptide (influenza MP; GILGFVFTL), additional control HLA-A*0201–restricted peptides were from Epstein-Barr virus latent membrane protein 2 (EBV LMP-2; CLGGLLTMV) and human immunodeficiency virus 1 (HIV-1) gag (SLYNTVATL).
For some experiments, TRs containing PBMCs (from day-7 samples) were depleted of CD8+ T cells with use of immunomagnetic beads (Miltenyi Biotec, Auburn, CA) before they were added to the cocultures. For some experiments, the TRsamples were separated from the recovery specimens by a transwell to check for soluble suppressor factors. In these cultures, APCs were added on either side of the transwell. In some experiments, the cocultures of TRs and recovery cells were performed in the presence of neutralizing anti–IL-10 antibody (10 μg/mL; R&D Systems, Minneapolis, MN) or 100 U/mL recombinant IL-2 (rIL-2; Chiron, Emeryville, CA).
Statistical analysis
The Student t test was used to compare results in different groups. The significance level was set atP < .05.
Results
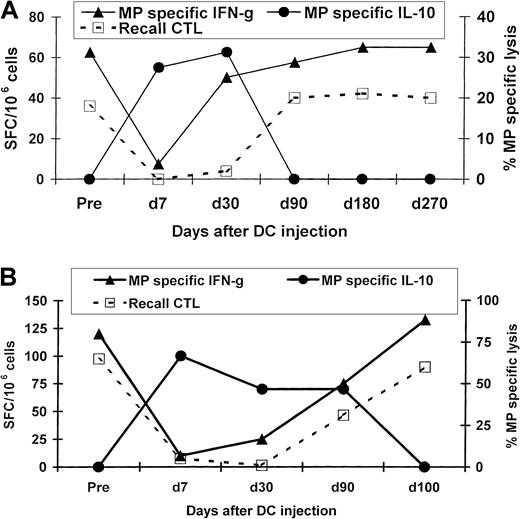
Both healthy volunteers had been primed to influenza at baseline because influenza MP–specific effector T cells were detectable on ELISPOT assays and peptide-specific CTLs could be expanded by a week of culture with mature DCs. However, 1 week after the injection of MP-pulsed immature DCs, these effector functions in the blood were silenced. This loss of function was reversible, with values returning to preinjection levels by 3 to 4 months after injection in both subjects (Figure 1). In a reciprocal fashion, silencing and recovery of effector T-cell function were associated with the appearance and then decline in peptide-specific IL-10 producers, which were no longer detectable after 90 to 100 days postimmunization (Figure 1). The DC injections were not associated with any clinical toxicity or clinical or serologic evidence of autoimmunity in either subject. Thus, the inhibition of effector T-cell function after a single injection of immature DCs was found to be self-limited.
Kinetics of the antigen-specific T-cell response after injection of influenza MP–pulsed immature DCs.
MP-specific T cells producing INF-γ, IL-10, and IL-4 were quantified in uncultured PBMCs by an ELISPOT assay. MP-specific lytic effectors were quantified after 7 days of culture with MP-pulsed DCs. Cytolysis data shown are for MP-specific lysis of T2 cells as targets (effector-to-target ratio, 20:1). (A) Results in subject Im2. (B) Results in subject Im1.
Kinetics of the antigen-specific T-cell response after injection of influenza MP–pulsed immature DCs.
MP-specific T cells producing INF-γ, IL-10, and IL-4 were quantified in uncultured PBMCs by an ELISPOT assay. MP-specific lytic effectors were quantified after 7 days of culture with MP-pulsed DCs. Cytolysis data shown are for MP-specific lysis of T2 cells as targets (effector-to-target ratio, 20:1). (A) Results in subject Im2. (B) Results in subject Im1.
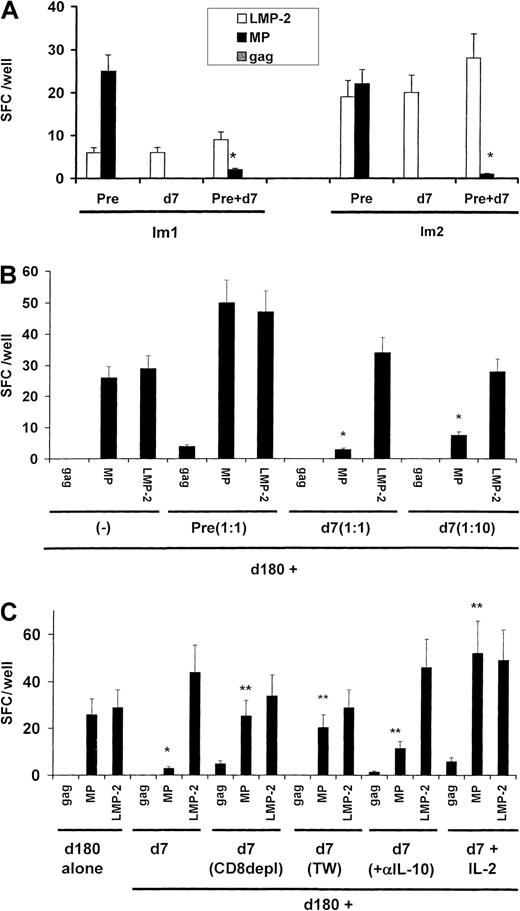
Because we had previously shown that the loss of circulating MP-specific effector T-cell function was not associated with a decline in circulating MHC tetramer–binding cells,22 we tested whether the effector silencing after injection of immature MP-pulsed DCs was mediated by the induction of TRs. To assess this directly, we mixed T cells from samples obtained 1 week after immunization (when the effector silencing was maximal) with samples obtained before immunization. The PBMCs obtained on day 7 inhibited MP-specific producers of INF-γ from cultures of preimmunization samples from both subjects (Figure 2A). The inhibition was specific for the immunizing peptide because responses to the control peptide LMP-2 were not silenced.
Suppressor assays.
(A) Presence of peptide-specific TRs cells in blood 7 days after injection. Blood mononuclear cells (2 × 105cells/well for subject Im1 and 3 × 105 cells/well for subject Im2) from preimmunization samples or samples obtained 7 days after immunization were cultured overnight, either separately or together (ratio, 1:1), in the presence of mature DCs pulsed with HLA-A*0201–restricted peptides from MP, LMP-2, and HIV gag at a DC/PBMC ratio of 1:60. Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay. The asterisk indicatesP < .05 for comparison with baseline reactivity on Student t test. (B) Dose-dependent inhibition of T-cell function. PBMCs (3 × 105 cells/well) from recovery specimens (day 180) from subject Im2 were mixed with preimmunization specimens (nonsuppressor) or various doses of specimens obtained on day 7 (suppressor; ratio of day 7 to day 180 samples, 1:1 or 1:10). Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay after overnight culture in the presence of DCs pulsed with influenza MP, EBV-LMP2, or HIV gag at a DC/PBMC ratio of 1:60. Data are representative of results from 2 similar experiments. The asterisk indicates P < .05 for comparison with baseline reactivity on Student t test. (C) Characterization of peptide-specific TRs. PBMCs (3 × 105cells/well) from recovery specimens (day 180) from subject Im2 were mixed (ratio, 1:1) with specimens from day 7, either unseparated, after CD8+ T cell depletion, or cultured physically separated in transwell cultures or in the presence of rIL-2 (100 U/mL). Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay after overnight culture in the presence of DCs pulsed with MP, EBV-LMP2, or HIV-gag at a DC/PBMC ratio of 1:60. Data are representative of results from 2 similar experiments. One asterisk indicates P < .05 for comparison with baseline reactivity on Student t test, and 2 asterisks indicateP < .05 for comparison with the suppressed reactivity.
Suppressor assays.
(A) Presence of peptide-specific TRs cells in blood 7 days after injection. Blood mononuclear cells (2 × 105cells/well for subject Im1 and 3 × 105 cells/well for subject Im2) from preimmunization samples or samples obtained 7 days after immunization were cultured overnight, either separately or together (ratio, 1:1), in the presence of mature DCs pulsed with HLA-A*0201–restricted peptides from MP, LMP-2, and HIV gag at a DC/PBMC ratio of 1:60. Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay. The asterisk indicatesP < .05 for comparison with baseline reactivity on Student t test. (B) Dose-dependent inhibition of T-cell function. PBMCs (3 × 105 cells/well) from recovery specimens (day 180) from subject Im2 were mixed with preimmunization specimens (nonsuppressor) or various doses of specimens obtained on day 7 (suppressor; ratio of day 7 to day 180 samples, 1:1 or 1:10). Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay after overnight culture in the presence of DCs pulsed with influenza MP, EBV-LMP2, or HIV gag at a DC/PBMC ratio of 1:60. Data are representative of results from 2 similar experiments. The asterisk indicates P < .05 for comparison with baseline reactivity on Student t test. (C) Characterization of peptide-specific TRs. PBMCs (3 × 105cells/well) from recovery specimens (day 180) from subject Im2 were mixed (ratio, 1:1) with specimens from day 7, either unseparated, after CD8+ T cell depletion, or cultured physically separated in transwell cultures or in the presence of rIL-2 (100 U/mL). Antigen-specific, INF-γ–producing cells were quantified by an ELISPOT assay after overnight culture in the presence of DCs pulsed with MP, EBV-LMP2, or HIV-gag at a DC/PBMC ratio of 1:60. Data are representative of results from 2 similar experiments. One asterisk indicates P < .05 for comparison with baseline reactivity on Student t test, and 2 asterisks indicateP < .05 for comparison with the suppressed reactivity.
Further characterization of the suppression was carried out only in samples from Im2, from whom we had additional cells available. The suppression of T-cell function was not due simply to competition for APCs or consumption of IL-2, since it was specific for immunizing peptide and observed only when suppressor samples (day 7) but not nonsuppressor (preimmunization) samples were added to the recovery samples (day 180; Figure 2B). T-cell suppression was dose dependent and observed even when the ratio of day-7 to day-180 cells was 1:10. Suppression was lost if day-7 cells were depleted of CD8+ T cells or if cell contact between day-7 and recovery T cells was prevented in transwell cultures (Figure 2C). Although the day-7 specimens had been shown to contain MP-specific IL-10 producers, addition of neutralizing anti–IL-10 antibody led to only a slight recovery of MP-specific effectors. However, the suppression was fully reversed by addition of 100 U/mL rIL-2. Thus, we found that peptide-specific CD8+ TRs induced in vivo by immature DCs inhibit CD8+ T cells in a cell-contact–dependent manner, that is, a manner largely independent of IL-10.
Discussion
These data provide direct evidence for the existence of antigen-specific CD8+ T-cell–mediated immune regulation and of the induction of such T cells in vivo in humans by immature DCs. Once induced, these cells have a limited life span in the circulation. Thus, naturally occurring TRs may require continued antigen presentation by trafficking immature DCs. Because peptide-specific, IL-10–producing cells are also induced by immature DCs, we refer to these suppressor cells as TRs, in keeping with previously established nomenclature. The regulation we observed required cell-cell contact and was largely independent of IL-10. These features are similar to those of CD4+ TRs induced by immature DCs in vitro.21 A subset of CD8+CD28− suppressor T cells that mediate suppression in a cell-contact–dependent fashion has also been described.12-14
The site where immature DCs generate TRs in vivo is not known. One possibility is that the DCs might traffic to lymph nodes to meet T cells recirculating by means of high endothelial venules. An alternative, which we favor because TRs have an activated phenotype, is that the DCs activate TRs that circulate from blood to extravascular spaces (here, the skin) and then return to the lymph node by means of the lymphatics. Although our studies help to confirm the presence of TRs and IL-10–producing cells in the CD8 compartment, compared with previously described CD4+ TRs, further work is needed to clarify their relation to CD25+ suppressor cells and their mechanism of action.
Our data suggest DC maturation as a key therapeutic target for the regulation of immunity.19 The inhibition of maturation in antigen-capturing DCs may promote the induction of TRs in vivo. Impairment of CD8+ T-cell suppressor function has been observed in patients with human autoimmune diseases such as lupus and multiple sclerosis.15,16 A role for TRs in acceptance of human allografts has also been suggested.24 In a reciprocal fashion, reduction of TRs may improve resistance to cancer and chronic infections, as was observed in a study of experimental tumors in mice.25
We thank Joseph Krasovsky for excellent technical assistance.
Supported in part by an investigator award from the Cancer Research Institute and grants from the National Institutes of Health (CA81138 to M.V.D., CA84512 to R.M.S., and MO-1-RR00102 to the Rockefeller General Clinical Research Center).
R.M.S. has a financial interest in Merix Biosciences, whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Madhav Dhodapkar, Laboratory of Tumor Immunology and Immunotherapy, Rockefeller University, 1230 York Ave, New York, NY, 10021; e-mail: dhodapm@mail.rockefeller.edu.