Abstract
The hematopathology subcommittee of the Mouse Models of Human Cancers Consortium recognized the need for a classification of murine hematopoietic neoplasms that would allow investigators to diagnose lesions as well-defined entities according to accepted criteria. Pathologists and investigators worked cooperatively to develop proposals for the classification of lymphoid and nonlymphoid hematopoietic neoplasms. It is proposed here that nonlymphoid hematopoietic neoplasms of mice be classified in 4 broad categories: nonlymphoid leukemias, nonlymphoid hematopoietic sarcomas, myeloid dysplasias, and myeloid proliferations (nonreactive). Criteria for diagnosis and subclassification of these lesions include peripheral blood findings, cytologic features of hematopoietic tissues, histopathology, immunophenotyping, genetic features, and clinical course. Differences between murine and human lesions are reflected in the terminology and methods used for classification. This classification will be of particular value to investigators seeking to develop, use, and communicate about mouse models of human hematopoietic neoplasms.
Introduction
The Mouse Models of Human Cancers Consortium (MMHCC) was established by the National Cancer Institute (NCI), National Institutes of Health, to accelerate the tempo at which mouse models of cancer are developed and validated by the scientific community. The Consortium aims to use these models to support discovery of new cancer-related genes and to disclose the pathways and processes through which these genes affect human cancer development, promote tumor progression, and facilitate metastasis. Ultimately, the models will be used to test new approaches to diagnosis and medical care for cancer.1
The hematopathology subcommittee of the MMHCC noted that terminology for hematopoietic neoplasms is inconsistently applied to lesions of mice. For example, similar abnormalities may be described as leukemia in one publication but as myeloproliferative disease in a publication from a different laboratory, thereby hampering the ability of the scientific community to draw general conclusions from the studies. The subcommittee recognized the need for a classification of murine hematopoietic neoplasms that would allow investigators to diagnose lesions as well-defined entities according to accepted criteria. Such a uniform classification will enhance the value of the scientific literature and will make it possible to meaningfully compare and contrast murine lesions with human lesions. Proposals for classification will also enhance the utility of the public database of murine hematopoietic lesions being created by MMHCC (http://emice.nci.nih.gov/. Accessed April 2, 2002) and the Jackson Laboratory (Bar Harbour, ME) under a grant from the NCI (http://tumor.informatics.jax.org/FMPro?-db=TumorInstance&-format=mtdp.html&-view). As a result of these efforts, it is hoped that (1) diagnoses stated in research publications will be based upon a community standard and (2) new contributions to the database will permit these classification proposals to be revised and enhanced.
The hematopathology subcommittee set out to develop consensus recommendations for classification of murine hematopoietic neoplasms and related disorders. In so doing, we have sought to parallel the structure of the World Health Organization (WHO) classification of human disorders2 but, where appropriate, to use different terminology in order to highlight differences between mouse and human biology. As with the WHO classification, these proposals make use of clinical/biologic, morphologic, immunophenotypic, and genetic characteristics of neoplasms for purposes of classification. It is hoped that the classification will be particularly relevant for analyses of genetically engineered mice, in which multiple animals with similar disease may be evaluated. The classification presented here is aimed specifically at researchers, pathologists, and other investigators seeking to develop and use mouse models of human nonlymphoid hematopoietic neoplasms. However, in the development of recommendations, there is an explicit recognition of the limitations of this classification when not all data are available. Finally, the MMHCC has developed a structure for the ongoing development of these classification proposals.
Development of the proposals
The subcommittee pursued its goals through a series of meetings and electronic communications. In an initial meeting, the state of classification and characterization of murine hematopoietic neoplasms was reviewed. Following this, the MMHCC and the Leukemia and Lymphoma Society (United States) jointly sponsored a meeting to discuss the biology of available mouse models of hematopoietic neoplasms. In October 2000, a meeting of medical hematopathologists, veterinary pathologists, investigators who use mouse models of hematopoietic neoplasms, and investigators with expertise in murine hematology and immunology was held near Bethesda, MD, under the auspices of the NCI. At this meeting, a broad spectrum of murine hematopoietic neoplasms was discussed, and these diseases were compared with their human counterparts. Areas were identified in which additional studies would be helpful to allow meaningful comparisons between murine and human neoplasms. Views for consensus classification were discussed. Following this meeting, proposals for classification of nonlymphoid hematopoietic neoplasms were drafted. These proposals were placed into an interactive Web site that allowed participants and interested colleagues to comment. These initial proposals were revised in light of these comments and are presented here. Primary materials as well as a broad body of published work were used in developing this classification. A summary of materials used by the subcommittee can be viewed in the on-line supplement.
Information needed for classification
Histopathology of mouse tissues as assessed by microscopic examination of paraffin-embedded tissues is central to pathologic diagnosis, including diagnosis of hematopoietic neoplasms. However, additional information is needed to permit rational comparisons between diseases of humans and mice. Peripheral blood findings (including blood cell counts and cytology) as well as bone marrow cytology play a critical role in the diagnosis of both mouse and human nonlymphoid hematopoietic neoplasms. Lineage assessment with cytochemical stains, immunohistochemical stains, and flow cytometric immunophenotyping provides valuable information. In addition, for diagnosing leukemia, it can be useful to perform serial examinations of peripheral blood and/or to assess whether a lesion is rapidly fatal upon transplantation. At present, genetic characterization of mouse lesions plays a limited but important role in classification.
Space constraints do not permit a full presentation of committee recommendations for approaches to characterization of lesions, but these recommendations are provided on the Blood website; see the Supplemental Data Set link at the top of the online article. This online information includes a practical guide to applying these classification proposals and will be of particular use to those new to the field of mouse hematopathology, whether they are studying spontaneous or induced models of disease. Also included in the on-line supplement are answers to questions generated during the development of the proposals.
Proposals
Overview
The committee sought to design a classification that can be readily compared with the human WHO classification but that also appropriately delineates the diseases as they occur in mice. For this reason, it was recommended that 4 categories of nonlymphoid hematopoietic neoplasms be defined: nonlymphoid leukemias, nonlymphoid hematopoietic sarcomas, myeloid dysplasias, and myeloid proliferations (nonreactive).
Diseases represented in these 4 categories are heterogeneous. Guidelines for subclassification reflect an effort to identify analogies with human diseases while at the same time permitting understandable, reproducible, and useful definition of diseases in mice. Gaps exist where diseases corresponding to diseases in the human classification have not yet been clearly described in mice. We anticipate that these gaps will be filled as scientists continue to develop and study additional mouse models of human cancer. It is recommended that investigators classify murine nonlymphoid hematopoietic neoplasms according to these proposals; they can also state that a disease has features of a particular human entity.2 For example, the disease arising inhMRP8-PMLRARA transgenic mice could be described as “acute myelogenous leukemia (AML) without maturation, with features of human acute promyelocytic leukemia.” Such assertions may then be assessed on their merits, rather than on the basis of concerns over the words used for classification.
The aim of these proposals is to provide recommendations for classification, not to serve as a comprehensive guide to the characterization of mouse hematopoiesis. The reader is therefore referred to a number of excellent references containing additional useful information and images.4-8
Nonlymphoid leukemia
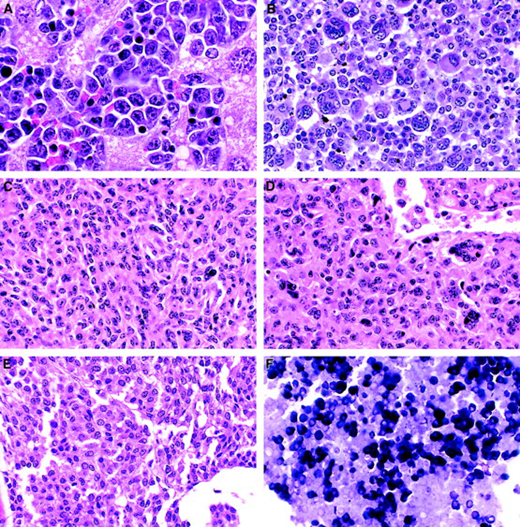
Variable appearance of myeloid leukemia in mice.
(A) MPD-like myeloid leukemia. Note complete maturation of myeloid forms to segmented neutrophils (Wright Giemsa, × 1000). Myeloproliferative disease can exhibit a similar morphology but lacks the constellation of other features that characterize leukemias. (B) Myeloid leukemia with maturation showing macrophages dispersed among the tumor cells (hematoxylin and eosin [H&E], × 100). (C) Myeloid leukemia with maturation. Note heterogeneous differentiation and large macrophages with striated eosinophilic cytoplasm (H&E, × 400). (D) Myeloid leukemia with maturation with many ring forms (H&E, × 1000). (E) Myeloid leukemia without maturation. Note many immature forms/blasts with azurophilic granules (Wright Giemsa, × 1000). (F) Myeloid leukemia without maturation seen as a poorly differentiated tumor in the splenic red pulp. No evidence of myeloid maturation can be seen. Residual nucleated erythroid cells are present (H&E, × 400).
Variable appearance of myeloid leukemia in mice.
(A) MPD-like myeloid leukemia. Note complete maturation of myeloid forms to segmented neutrophils (Wright Giemsa, × 1000). Myeloproliferative disease can exhibit a similar morphology but lacks the constellation of other features that characterize leukemias. (B) Myeloid leukemia with maturation showing macrophages dispersed among the tumor cells (hematoxylin and eosin [H&E], × 100). (C) Myeloid leukemia with maturation. Note heterogeneous differentiation and large macrophages with striated eosinophilic cytoplasm (H&E, × 400). (D) Myeloid leukemia with maturation with many ring forms (H&E, × 1000). (E) Myeloid leukemia without maturation. Note many immature forms/blasts with azurophilic granules (Wright Giemsa, × 1000). (F) Myeloid leukemia without maturation seen as a poorly differentiated tumor in the splenic red pulp. No evidence of myeloid maturation can be seen. Residual nucleated erythroid cells are present (H&E, × 400).
Erythroid leukemia, megakaryocytic leukemia, histiocytic sarcoma, and mast cell sarcoma in mice.
(A) Erythroid leukemia. Note erythroblasts in liver (H&E, × 1000). (B) Megakaryocytic leukemia. Note various forms of immature neoplastic megakaryocytes (H&E, × 400). (C) (D) Histiocytic sarcoma: a relatively common tumor in mice that is rare in humans (H&E, × 400). (C) A solid sheet of eosinophilic histiocytes forming elongated or round patterns is seen. (D) Histiocytic sarcoma showing multinucleated histiocytes and vascular invasion in the liver. (E) A mast cell sarcoma showing a uniform population of small cells with prominent granular eosinophilic cytoplasm (H&E, × 400). (F) Giemsa stain of mast cell sarcoma shows strong metachromasia of many tumor cells. Metachromatic staining is also revealed by toluidine blue stain (Giemsa, × 400).
Erythroid leukemia, megakaryocytic leukemia, histiocytic sarcoma, and mast cell sarcoma in mice.
(A) Erythroid leukemia. Note erythroblasts in liver (H&E, × 1000). (B) Megakaryocytic leukemia. Note various forms of immature neoplastic megakaryocytes (H&E, × 400). (C) (D) Histiocytic sarcoma: a relatively common tumor in mice that is rare in humans (H&E, × 400). (C) A solid sheet of eosinophilic histiocytes forming elongated or round patterns is seen. (D) Histiocytic sarcoma showing multinucleated histiocytes and vascular invasion in the liver. (E) A mast cell sarcoma showing a uniform population of small cells with prominent granular eosinophilic cytoplasm (H&E, × 400). (F) Giemsa stain of mast cell sarcoma shows strong metachromasia of many tumor cells. Metachromatic staining is also revealed by toluidine blue stain (Giemsa, × 400).
The choice of the term “nonlymphoid leukemia” rather than “myeloid leukemia” as a broad category allows for the useful classification of “myeloid” (granulocytic/monocytic) as distinct from erythroid, megakaryocytic, and biphenotypic leukemias. It was believed that the omission of the term “acute” from the broad category would make possible the inclusion of both aggressive diseases with numerous immature forms/blasts and aggressive diseases with maintained differentiation. This is desirable because of otherwise often similar features of these diseases in mice. There was broad consensus that leukemias are characterized by cytopenias and by increased nonlymphoid hematopoietic cells in bone marrow and spleen. In addition, there was consensus that leukemias are characterized by evidence of dissemination of neoplastic cells. While most cases of leukemia are associated with the spread of nonlymphoid hematopoietic cells outside of the blood, spleen, and bone marrow, some leukemias present with minimal spread but with a high white blood cell count and numerous immature forms/blasts in the blood (see, eg, Cuenco et al9). For this reason, alternative criteria for defining dissemination are included in the definition. Finally, after discussion of how to use the percentage of immature forms/blasts (see on-line glossary for definition and discussion of the term “immature forms/blasts”), tempo of disease course, and transplantability to distinguish leukemia from less aggressive disturbances of hematopoiesis, alternative criteria for defining a process as “leukemia” were agreed upon. The first of these (5A, at least 20% immature forms/blasts) is straightforward, is closely aligned with the definition of acute leukemia in the WHO classification, and should be readily available to the examining pathologist. The other criteria (5B1, 5B2) reflect our opinion that diseases characterized by rapid lethality in the primary animal or in secondary recipients should also be considered to be “leukemias” when this information is available (which it will be for many studies of genetically engineered mice). It is recommended that “acute” be used to describe a nonlymphoid leukemia only when the disease fulfills both criterion 5A and either criterion 5B1 or criterion 5B2.
The characterization needed for diagnosis and subclassification of leukemias extends beyond histopathology; it is recognized that the modalities required for assessing the criteria included in these proposals may not be available to the pathologist or scientist evaluating an animal. In such cases, the investigator should note the limitations and should state the diagnosis consistent with available findings. The recommendations of Dunn11 and others4,6,8,12 13 regarding histopathologic diagnosis of leukemia are pertinent, even in circumstances in which these suggestions may lead to presumptive rather than definitive diagnosis. Additional studies of the features of murine nonlymphoid hematopoietic illnesses that correlate with aggressive biologic behavior may allow other criteria to be developed that will aid in diagnosis of nonlymphoid leukemia.
Neoplasms that in humans would be confined mainly to the bone marrow often also involve the splenic red pulp in mice. This occurs because the spleen is an important hematopoietic organ throughout its life. The splenic hematopoietic tissue has a capacity to expand that is not available to the hematopoietic tissue of the medullary cavity in mice or in humans. This may result in less marked competition between neoplastic and nontransformed hematopoietic counterparts in mouse leukemias and may account for neutropenia being a less common feature of leukemias of mice than of humans.
Because the mouse spleen is a hematopoietic organ, diagnosis and subclassification of nonlymphoid leukemias must take into account the following: (1) the possibility of leukemias arising in the spleen, (2) the mixture of cell types present in the spleen as well as the bone marrow, and (3) the capacity of normal splenic hematopoietic tissue to expand. Consideration of these issues led to the following recommendations. First, the specification of at least 20% immature forms/blasts in the spleen has been included as part of criterion 5A for nonlymphoid leukemia. For the purpose of diagnosis of leukemia, all nucleated spleen cells should be included when this criterion is applied. Second, although leukemias can arise in the spleen, the bone marrow is often diffusely involved at the time these leukemias are diagnosed and studied. When this is the case, bone marrow should be used as the primary tissue for subclassification because these marrows are overwhelmingly composed of leukemic cells whereas the spleens often contain a mixture of leukemic cells and normal hematopoietic elements. Third, in some cases it may be necessary to subclassify leukemias on the basis of findings in the spleen, including (1) leukemias of splenic origin early in their course and (2) leukemias for which bone marrow is fibrotic or otherwise not available for evaluation. In these cases, the guidelines set forth in Figure 2for the purpose of subclassification of nonlymphoid leukemias can be applied to the spleen with these provisions: (1) Lymphocytes should be excluded from consideration when immature forms/blasts and erythroid, megakaryocytic, monocytic, and neutrophilic cells are enumerated; and (2) whereas at least 20% monocytic cells in spleen (lymphocytes excluded) can be used to define a monocytic component, caution is warranted in similarly using at least 20% neutrophilic cells in spleen (lymphocytes excluded) to define a neutrophilic component because such cells may represent residual normal hematopoietic tissue.
As with the subclassification of human AML in the WHO classification, it is recommended that investigators note if multilineage dysplasia is present and if the illness is therapy related.
Nonlymphoid hematopoietic sarcoma
In the human WHO classification, histiocytic sarcoma is considered one of the histiocytic/dendritic cell neoplasms; mast cell sarcoma is considered with mast cell leukemia as one of the mast cell diseases; and myeloid sarcoma is considered an alternative presentation of acute myeloid leukemia. The broad spectrum of described nonlymphoid hematopoietic neoplasms in humans and an understanding of their behavior over time led to this system for classification.
Nonlymphoid leukemias and nonlymphoid hematopoietic sarcomas are closely related diseases in mice, as they are in humans. Nevertheless, the presentation and appearance of these diseases are generally distinct in the mouse. Furthermore, our understanding of the evolution of these diseases over time is limited. It therefore appears appropriate to consider nonlymphoid hematopoietic sarcoma as a separate category of disease in mice. In addition to the information contained here, the reader is referred to previous publications that describe the histopathology of histiocytic sarcomas and mast cell sarcomas.5 6
It is recognized that these sarcomas may progress to leukemia, particularly upon serial transplantation, and that there are cases that are difficult to classify even at presentation. Of note, a diagnosis of sarcoma can occasionally be made in animals with a coexistent leukemia or lymphoma distinct from the sarcoma. For example, mice withBCR-ABL–induced leukemias may also haveBCR-ABL–induced histiocytic sarcomas.14
Myeloid dysplasia
The need for a term to encompass diseases that are characterized by abnormal differentiation and cytopenias was apparent. The term “myeloid dysplasia” was chosen rather than myelodysplastic syndrome (MDS) to allow the inclusion of mouse diseases that, in principal, are closely related to diseases designated MDS in humans, while avoiding the implication that these mouse diseases necessarily correspond to entities described in the WHO classification.
Current knowledge of murine diseases that may be classified as myeloid dysplasias is limited, hampering our ability to provide guidelines for diagnosis and subclassification. Nevertheless, if a disease does not meet criteria for nonlymphoid leukemia, the criteria for myeloid dysplasia encompass a range of diseases with abnormal differentiation and cytopenias. The view that guided criterion 2 is that both dyspoiesis and increased nonlymphoid immature forms/blasts can reflect abnormal differentiation. In regard to criterion 2B, a cutoff of 20% was chosen to set a high threshold for calling diseases “myeloid dysplasia” specifically when morphologic dyspoiesis is not observed. Diseases characterized by (1) cytopenias and dyspoiesis or (2) cytopenias, dyspoiesis, and increased immature forms/blasts (even when the immature forms/blasts are fewer than 20%) may of course also be classified as “myeloid dysplasia.” Note that most murine neoplasms with at least 20% immature forms/blasts are leukemias and not myeloid dysplasias.
The difficulty of classifying human diseases that have features of both MDS and MPD was reflected in the WHO classification by the creation of a category designated myelodysplastic/myeloproliferative disease. In making recommendations for mice, we intended the term “myeloid dysplasia” for diseases that are more closely related to human MDS than either human MPD or MD/MPD. This view guided criterion 1.
At this time, recommendations regarding the morphologic features that should be considered dyspoiesis in mice are speculative. Characteristics described as dyspoiesis in cats and dogs as well as those described in the French-American-British proposals for classification of human MDS may be applicable to mice.15 16 For erythroid cells, dyspoiesis may include maturation of the cytoplasm preceding maturation of the nucleus (megaloblastic change), nuclear fragmentation, irregular nuclear contours, abnormal mitotic figures, ringed sideroblasts, and multiple nuclei. For megakaryocytes, dyspoiesis may include cells with multiple separated nuclei, small cells with one or more small oval nuclei in mature cytoplasm (micromegakaryocytes), large cells with unlobated nuclei, and large cells with bizarre hypersegmentation. For neutrophilic cells, dyspoiesis may include abnormal cytoplasmic maturation, manifested as pale basophilic cytoplasm in mature neutrophils, and abnormal nuclear maturation as evidenced by the presence of open chromatin in mature cells. In addition, we have noted that the presence of neutrophils with lobated, as opposed to ring-shaped, nuclei may represent dysplasia.
In humans, there are patients with MDS who exhibit minimal morphologic dyspoiesis. Similar diseases may also exist in mice that should be classified as myeloid dysplasias. A monoclonal population of hematopoietic cells, a karyotypically abnormal population for example, can support a diagnosis of myeloid dysplasia when morphologic dyspoiesis is minimal.
In regard to subclassification, we make the following provisional recommendations. First, the cell types that are dysplastic or decreased should be stated. Second, if a disease is characterized by increased immature forms/blasts without morphologic dyspoiesis, it may be designated a “cytopenia with increased blasts.” Third, if a disease is characterized by morphologic dyspoiesis, it may be designated a “myelodysplastic syndrome.” If a disease has features of a particular human myelodysplastic syndrome, it may be designated a “myelodysplastic syndrome with features of a named human MDS.”
Myeloid proliferation (nonreactive)
The term “myeloproliferative disease” has been used to describe modest increases in bone marrow and splenic granulocytic cells in certain strains of genetically engineered mice, rapidly fatal neoplasms with large numbers of predominantly differentiating cells, and everything in between. For purposes of classification, we thought it would be helpful to distinguish between aggressive proliferations and indolent processes. It was furthermore thought that the term “myeloproliferative disease” should be used for disorders that parallel those in the WHO classification, as opposed to being a general term for processes with increased numbers of nonlymphoid hematopoietic cells. We propose that aggressive neoplasms with retained differentiation be classified as MPD-like myeloid leukemias according to the criteria presented in Figure 2. In addition, the term “myeloid proliferation (nonreactive)” is proposed to encompass (1) diseases similar to human MPD as well as (2) mouse disorders that lack a human counterpart but are more similar to MPD than to other human illnesses.
Myeloid proliferations (nonreactive) include a wide spectrum of processes, with those similar to human diseases at one end and subtle increases in splenic nonlymphoid hematopoietic cells, most often in genetically engineered mice, at the other end. The possibility of considering subtle expansions as entities outside the classification of nonlymphoid hematopoietic neoplasms was considered, but was rejected for 2 reasons. First, these expansions can give rise to leukemia. Second, they often result from the expression of genetic abnormalities closely associated with human leukemias. The term “myeloid proliferation (nonreactive)” was chosen rather than myeloproliferative disease to allow the inclusion of such mouse processes while avoiding the implication that these abnormalities necessarily correspond to particular entities described in the WHO classification. In making recommendations for mice, we intend the term “myeloid proliferation” for processes that are closely related to either human MPD or MD/MPD, as well as for nonreactive, persistent, genetically determined expansions that may progress to overt disease.
Mouse diseases that are classifiable as myeloid proliferations have been described, but the extent to which these diseases can be reproducibly and usefully subclassified is unclear. At present, we make the following provisional recommendations. First, the cell types that are increased should be stated. Second, if a disorder is limited to increased nonlymphoid hematopoietic cells in spleen and/or bone marrow without increased counts in the peripheral blood, it may be designated a “myeloproliferation (genetic).” Third, if a disease is characterized by increased blood counts along with increased nonlymphoid hematopoietic cells in spleen and/or bone marrow, it may be designated a “myeloproliferative disease.” If a disease has features of a particular human chronic myeloproliferative disease or myelodysplastic/myeloproliferative disease, it may be designated a “myeloproliferative disease with features of a named human MPD or MD/MPD.”
Distinguishing neoplasms from reactive conditions
Reactive abnormalities of hematopoietic cells can result from various conditions, including nutritional deficiencies, infections, tumors of nonhematopoietic cells, toxins, autoimmune blood cell destruction, and dysregulation of the lymphoid system. Investigators need to be alert to the possibility that an expansion of hematopoietic cells represents a reactive, as opposed to a neoplastic, process, and should be especially cautious in making a diagnosis of hematopoietic neoplasm whenever an infection or a nonhematopoietic tumor is present. Two types of reactive processes that are common in mice can be particularly difficult to distinguish from neoplasms: extramedullary hematopoiesis, especially as reflected by increased hematopoiesis in the spleen, and leukemoid reactions in the peripheral blood. Evidence for a process being neoplastic includes the following: the absence of infectious or inflammatory lesions; the absence of nonhematopoietic tumors; adequate nutrition; the absence of exposure to drugs or toxins; the abnormalities' being persistent and progressive as assessed by serial physical examination or examination of the blood; presence of clonal hematopoiesis (see below); and the lesion's being transplantable (see below).
Monoclonality
Neoplasms typically arise from the sequential acquisition of genetic mutations and therefore are generally monoclonal proliferations. However, it was noted that if genetic changes engineered into mice are sufficient to induce disease, then such disease will not be monoclonal. In addition, assessment of clonality for nonlymphoid hematopoietic neoplasms requires the application of methods that may not yet be widely available or may not be applicable (eg, cytogenetic analysis, X-chromosome inactivation, assessment of provirus integration status). It was further noted that monoclonal proliferations may not necessarily cause illness. For proliferations of nonlymphoid hematopoietic cells, clonality can be used to help indicate that a lesion is neoplastic, but assessment of clonality is not required for diagnosis or classification.
Transplantation
The ability of expanded populations of hematopoietic cells to transplant is an important biologic characteristic. Demonstration that a disease is transplantable can be particularly valuable in helping to exclude the possibility that a lesion is reactive. However, although transplantation seems to correlate with degree of malignancy, some benign tumors can be transplanted whereas lesions that behave in a malignant fashion in the primary host are not necessarily transplantable. Given current limitations in our understanding of the relationship between transplantability, biologic behavior, and human neoplasms, we have proposed that transplantability per se should not serve as a defining element for mouse hematopoietic neoplasms.
To expand our understanding of the significance of transplantability, it is recommended that investigators include descriptions of the cells transplanted (origin, preparation, number); route of injection; type of recipient (eg, nude/other immunodeficient strain/histocompatible, unirradiated/histocompatible, sublethally irradiated/histocompatible, lethally irradiated); and character of disease in the recipient (localized/disseminated, time from transplantation until illness). It was noted that tail vein injection of 1 × 106 cells into sublethally irradiated histocompatible recipients is one accepted method for assessing transplantability of nonlymphoid hematopoietic lesions.17
Perspectives
Previous proposals for the classification of murine nonlymphoid hematopoietic neoplasms have been described by Dunn,11Perkins,4 and, more recently, by Fredrickson and Harris6 and Frith et al.8 The Bethesda proposals build upon these previous categorizing schemes to guide the classification of the wide spectrum of nonlymphoid hematopoietic neoplasms that develop in genetically engineered mice. These proposals will also permit newly described diseases to be placed into an existing framework.
The MMHCC hematopathology subcommittee is continuing its work and we look to the broader research community for input related to these efforts. This classification will be revised and updated as additional information becomes available. Further development is essential. Investigators are invited to submit their e-mail addresses to the subcommittee in order to receive requests for information, updates to this classification, and postings to the MMHCC Web site of protocols for characterization as well as descriptions of new models. The Web site currently reflects ongoing efforts to place known models of nonlymphoid hematopoietic neoplasms into the diagnostic groups delineated here. In the future, the MMHCC plans to provide additional information about and access to murine models of nonlymphoid hematopoietic neoplasms.
Supported by funding from the National Cancer Institute for the meetings of the Mouse Models of Human Cancers Consortium hematopathology subcommittee; by grants CA84221 and CA75986 from the National Institutes of Health (S.C.K.); and in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. NO1-CO-56000; S.C.K. is a recipient of a Burroughs Wellcome Fund Career Award and is the 32nd Edward Mallinckrodt Junior Scholar.
The online version of the article contains a data supplement.
Scott C. Kogan, UCSF Comprehensive Cancer Center, 2340 Sutter St, Rm N-361, Box 0128, San Francisco, CA 94143; e-mail: skogan@cc.ucsf.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Cheryl Marks, Susan Seweryniak, and R. Allan Mufson for their support of this project; Kevin Shannon, H. Jeffrey Lawrence, and Richard A. Van Etten for helpful discussions; and Philip Chan for his assistance. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.