B-cell precursors are present in the thymus, and the thymic microenvironment is the source of lymphopoietic factors that include interleukin-7 (IL-7). Despite the fact that intrathymic B-cell progenitors are bone marrow–derived cells, the data in this report demonstrate that these progenitors accumulate at an early pro–B-cell stage of development, cycle less than their bone marrow counterparts, and fail to differentiate efficiently. Additional studies presented herein indicate that these effects are mediated, at least in part, by soluble factors produced by the thymic microenvironment and suggest that they affect the ability of pro–B cells to respond optimally to IL-7. Taken together, these observations demonstrate a specific inhibition of intrathymic B lymphopoiesis, which in turn may explain why lymphoid cell production in the thymus is largely restricted to production of T-lineage cells despite the fact that B-cell precursors and B-lymphopoietic stimuli are present in that organ.
Introduction
Sustained T-cell development in the thymus is thought to be dependent on continuous migration of bone marrow–derived precursors to that organ.1-4 While T lineage–committed progenitors could be included among these cells,5experimental evidence suggests that at least some intrathymic lymphoid precursors are multipotent and retain the capacity to generate B cells.6,7 The recently characterized bone marrow (BM) common lymphoid precursor (CLP),8 which has B- and T-cell developmental potential, could be the BM population that migrates to the thymus and sustains thymopoiesis.
Despite the fact that B-cell precursors are present in the thymus, B lymphopoiesis in that organ is minimal. One reason for this is that entry of lymphoid precursors into the B lineage may be limited. In this regard, it has been proposed that binding of the Notch1 receptor on multipotential lymphoid precursors, such as the CLP, to ligands expressed by thymic stromal cells9 results in commitment to the T lineage and a block in B-cell development.10-12 Activation of Notch1 signaling pathways may be a critical means by which entry of multipotential precursors into the B-cell lineage is blocked within the thymus. However, some thymic lymphoid precursors may fail to do so, thereby allowing pro–B cells to develop. Indeed, it has been reported that 10% to 13% of cells within the CD3−CD4−CD8− triple-negative (TN) pool of intrathymic progenitors express the CD45R (B220) B lineage–associated cell surface determinant.13
Nevertheless, B-lineage cells in the thymus account for less than 1% of total lymphocytes.14-20 Such a low frequency is surprising, because B-cell precursors present among TN thymocytes are exposed to many of the same microenvironmental stimuli as their counterparts in the BM. For example, the interaction between lymphoid cells and stromal cells in both organs is mediated through similar ligand-receptor complexes, such as very late antigen-4/vascular cell adhesion molecule-1 (VLA-4/VCAM-1) and stromal cell–derived cytokines that include interleukin-7 (IL-7).21 IL-7 stimulates pro–B-cell proliferation and potentiates immunoglobulin heavy chain gene rearrangements in those precursors.22-24Because it has been established that they are bone marrow–derived cells,25 intrathymic B-cell progenitors would be expected to respond to IL-7 and other lymphopoietic factors.
These observations suggest that mechanism(s) that limit the maturation of intrathymic B-cell progenitors exist. Such regulatory pathway(s) would ensure that expansion of B-lineage cells able to compete for microenvironmental niches required for T lymphopoiesis does not occur. There may be additional reasons for limiting intrathymic B-cell production. A case has been made that thymic B cells are involved in selection of the T-cell repertoire.26 Thus, an inordinate increase in their numbers might be detrimental to the thymic education process. Therefore, it is likely that signals from the thymic microenvironment minimize the development of B-lineage cells that develop in that organ.
The data in this report support this hypothesis and demonstrate that B-cell progenitors in the thymus accumulate at an early pro–B-cell stage of development and fail to differentiate efficiently. These effects are consistent with findings demonstrating that intrathymic pro–B cells respond inefficiently to IL-7. Taken together, these results document an unappreciated inhibitory effect on B lymphopoiesis by the thymic microenvironment that helps to explain why lymphoid cell production in the thymus is largely restricted to production of T-lineage cells.
Materials and methods
Mice
BALB/cJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and housed in the vivarium of the University of California at Los Angeles (UCLA) Division of Laboratory Animal Medicine. Timed pregnant Swiss/Webster mice were purchased from Taconic Farms (Germantown, NY). Ifnar1−/− mice were bred and maintained in the vivarium of the University of Alabama, Birmingham, AL.
Preparation of cell suspensions
Single-cell suspensions of thymus were prepared by gently pushing tissues through a fine mesh screen into α-minimal essential medium (MEM; GIBCO, Grand Island, NY) supplemented with 5% fetal calf serum (FCS; Atlanta Biologicals, Atlanta, GA). BM cells were flushed from femurs using a syringe fitted with a 23-gauge needle. Cells were counted with a hemacytometer, and viability was determined by eosin dye exclusion.
Fetal thymic organ cultures
Fetal thymic organ cultures (FTOCs) were established according to the protocol described by Jenkinson et al.27 Briefly, fetal thymic lobes from 15-day-old Thy-1.1 Swiss/Webster embryos were harvested aseptically, dissected free from extraneous tissue, and cultured for 5 days in the presence of 1.35 mM deoxyguanosine to deplete endogenous thymocytes. The lobes were then rinsed and incubated with donor cells in hanging drop cultures in Terasaki plates (Fisher, Tustin, CA) for 48 hours. Subsequently, lobes were transferred to FTOC on filter/gelfoam rafts in RPMI 1640 supplemented with 10% FCS, 5 × 10−5 M 2-β mercaptoethanol, 100 U/mL streptomycin, and 100 U/mL penicillin and placed in a humidified, 5% CO2/air incubator at 37°C. Cell proliferation and differentiation in FTOC was assessed at regular intervals following culture initiation, as described below.
Long-term BM cultures
Long-term myeloid BM cultures (LTBMCs) were initiated as described by Dexter et al.28 Briefly, the contents of a femur were flushed into a 25 cm2 tissue culture flask in α-MEM supplemented with 20% horse serum and 10−6 M sodium hydrocortisone succinate. Cultures were incubated at 33°C in a 5% CO2/air incubator. After 2 weeks, cultures were recharged with 106 BM cells per flask. B-cell development was induced by switching cultures to the B-lymphoid permissive conditions (RPMI 1460 supplemented with 5% FCS and 5 × 10−5 M β-mercaptoethanol) described by K.D.,29 and by Whitlock and Witte.30
Preparation of BM and thymus stromal cells
Confluent, heterogeneous BM stromal cell cultures were established by treating LTBMCs with 5 μg/mL mycophenolic acid for 2 to 4 weeks to eliminate hematopoietic cells as described.31 The adherent stromal cells were then maintained in α-MEM supplemented with 10% FCS in a 37°C, 5% CO2/air incubator and were passaged minimally to retain the characteristics of primary stroma. Heterogeneous thymic stromal cell cultures were established as described.32 Thymuses were digested with trypsin/EDTA (ethylenediaminetetraacetic acid) and collagenase dispase in Ca++-, Mg++-free medium for 35 minutes at 37°C. The confluent thymic stromal cells were then fed weekly with MEM D-valine supplemented with 10% FCS and maintained in a 37°C, 5% CO2/air incubator. These cells were passaged minimally to retain the characteristics of primary stroma. The generation of the S17 stromal cell line has been described.33 The S17 BM stromal cell line was fed weekly with α-MEM supplemented with 10% FCS.
Diffusion chamber cultures
In some experiments, heterogeneous BM or thymic stromal cells were grown to confluency in transwells fitted with membranes containing 0.4-μm pores (Becton Dickinson, San Jose, CA). Inserts were inserted into wells of 6-well plates in which myeloid LTBMCs had been established. Cultures were then switched to B-lymphoid permissive conditions, fed twice weekly, and cells assayed for B-cell production by immunophenotyping.
Immunofluorescence and cell sorting
BM cell suspensions were treated with Tris-ammonium chloride (pH 7.2) to lyse red blood cells. Cells were then incubated with an anti-CD16/CD32 antibody (FcγRII/III, clone 2.4G2; Pharmingen, San Diego, CA) to reduce nonspecific labeling prior to staining. The following monoclonal antimouse antibodies used for staining were all obtained from Pharmingen: CD45R (B220, clone RA3-6B2), CD127 (IL-7Rα, clone SB/14), CD43 (clone S7), CD24 (HSA, clone 30-F1), and Ly-51 (BP-1, clone 6C3). These antibodies were conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), TriColor (TC), or biotin. Biotinylated antibodies were revealed with TC-conjugated streptavidin (Southern Biotechnology, Birmingham, AL). Optimal working dilutions were determined for each antibody prior to use. All incubations were performed in Ca++-, Mg++-free phosphate-buffered saline (PBS) at 4°C for 30 minutes. Following the last wash, 104 to 105 live cells per sample were analyzed by flow cytometry on a FACScan (Becton Dickinson) with CellQuest software (Becton Dickinson).
BM cells were incubated with FITC-conjugated anti-CD45R, PE-conjugated anti-CD43, or PE-conjugated anti-IgM, as described above, prior to sorting on a FACStarplus flow cytometer (Becton Dickinson) located in the Jonsson Comprehensive Cancer Center Flow Cytometry Core at UCLA. Reanalysis showed that the purity of the sorted CD45R+IgM− population was routinely above 99%, while we routinely achieved 95% purity for CD45R+CD43+cells. In the experiments described herein, 5 × 104CD45R+sIgM− or 2.5 × 104CD45R+CD43+ cells were seeded into fetal thymic lobes or onto S17 BM stroma.
Cell cycle analysis
Cells were fixed in 0.5% paraformaldehyde for 15 minutes at room temperature and then permeabilized with 70% ethanol and resuspended in Ca++-, Mg++-free PBS supplemented with 1 μg/mL 7-amino actinomycin D (7-AAD; Calbiochem, San Diego, CA). At least 105 cells were acquired with a FACScan, and their cycling status was estimated using Modfit LT software (Becton Dickinson).
Results
Thymic B-cell differentiation is blocked at an early stage in vivo
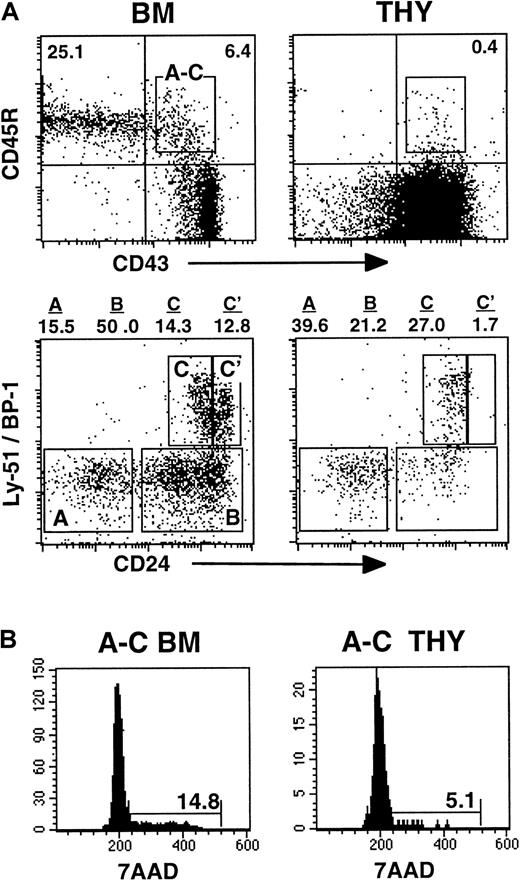
Multiple stages of B-cell differentiation in the BM can be defined based on the differential expression of various cell surface determinants. Because thymic B-lineage cells are bone marrow–derived,25 their expression of the CD45R, CD43, Ly51 (BP-1), and CD24 cell surface determinants was assessed by flow cytometry. This combination of reagents allows the pro–B-cell pool to be subdivided into fractions A, B, C, and C′, as defined by Hardy et al.34 Fraction A includes the most immature pro–B cells, while those in fraction C′ are completing immunoglobulin (Ig) heavy chain gene rearrangements before their transition into pre–B cells.
As expected, cells in the bone marrow pro–B-cell compartment were present at the frequency described previously35 and included populations that had matured to the C′ stage of development (Figure 1A). In contrast, very few thymic pro–B cells had matured to the fraction C′ stage (Figure 1A). Because the most actively proliferating B-lineage cells are those in the pro–B-cell compartment, the cell cycle status of intrathymic CD45R+CD43+ cells also was determined. As shown in Figure 1B, the frequency of cycling thymic pro–B cells was approximately 3-fold lower than that observed with comparable populations from the BM.
B-cell development in the thymus is blocked at the pro–B-cell stage.
(A) Phenotype of CD45R+CD43+ pro–B cells harvested directly from the bone marrow (BM) or thymus (THY) of BALB/c mice. The frequency of cells in fractions A, B, C, and C′ stages of development was assessed by analysis of Ly51 (BP-1) and CD24 expression on the gated CD45R+CD43+ cells. (B) Cell cycle status of pro–B cells in the BM and thymus. Data in the figures are representative of 3 experiments.
B-cell development in the thymus is blocked at the pro–B-cell stage.
(A) Phenotype of CD45R+CD43+ pro–B cells harvested directly from the bone marrow (BM) or thymus (THY) of BALB/c mice. The frequency of cells in fractions A, B, C, and C′ stages of development was assessed by analysis of Ly51 (BP-1) and CD24 expression on the gated CD45R+CD43+ cells. (B) Cell cycle status of pro–B cells in the BM and thymus. Data in the figures are representative of 3 experiments.
B-cell development is blocked in FTOC
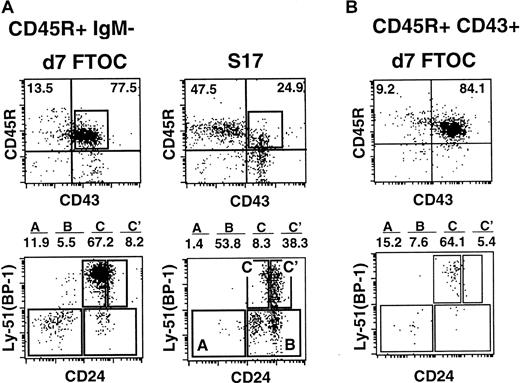
The above results indicate that B-cell production in the thymus is blocked at a relatively early stage of development. In order to assess the fate of isolated B-cell progenitors in the thymic environment in more detail, a modification of the FTOC system was used. Fetal thymic lobes from day-15 embryos were treated with deoxyguanosine to remove endogenous thymocytes prior to seeding with FACS-purified CD45R+sIgM− bone marrow cells. Thymic lobes prepared in this manner are fully functional and support T-cell development.36 Other aliquots of CD45R+sIgM− cells were seeded on the BM stromal cell line S17, which has been shown to support B-cell differentiation in vitro.33 Cells were recovered and analyzed phenotypically 7 days following initiation of these cultures, as described above.
Figure 2 demonstrates that while B-lineage cells were recovered from thymic lobes, they did not efficiently mature past the fraction C stage. These data are consistent with observations made on freshly harvested thymic pro–B cells (Figure1A) and indicate the validity of the FTOC system for analyzing thymic B lymphopoiesis. The fate of CD45R+ surface (s)IgM− cells seeded in FTOC contrasts with that of other aliquots of CD45R+sIgM− cells seeded onto the S17 stromal cells. In this case, cells that were recovered 7 days later had efficiently matured to the C′ stage of development (Figure 2A). Similar results were obtained when fetal thymic lobes were seeded with FACS-purified CD45R+CD43+ (fractions A-C) pro–B cells. Again, when lobes were processed 7 days after seeding, few pro–B cells had matured past the fraction C stage of development (Figure 2B).
Phenotype of CD45R+CD43+ pro–B cells following culture on S17 stromal cells or in FTOCs.
(A) 5 × 104 CD45R+sIgM− cells were seeded into FTOCs or onto S17 stroma. Cells were phenotyped 7 days later as described in Figure 1. (B) 2.5 × 104fluorescence-activated cell-sorter (FACS)–purified CD45R+CD43+ pro–B cells were seeded into FTOCs. Phenotypic analysis was performed on cells harvested from lobes 7 days after initiation of cultures. Data in the figures are representative of 3 experiments.
Phenotype of CD45R+CD43+ pro–B cells following culture on S17 stromal cells or in FTOCs.
(A) 5 × 104 CD45R+sIgM− cells were seeded into FTOCs or onto S17 stroma. Cells were phenotyped 7 days later as described in Figure 1. (B) 2.5 × 104fluorescence-activated cell-sorter (FACS)–purified CD45R+CD43+ pro–B cells were seeded into FTOCs. Phenotypic analysis was performed on cells harvested from lobes 7 days after initiation of cultures. Data in the figures are representative of 3 experiments.
Exposure to the thymic environment renders B-cell progenitors unresponsive to lymphopoietic signals
Because cytokines such as IL-7 are produced in the thymus,37 38 it seemed unlikely that failure of pro–B cells to mature was due to the absence of B-lymphopoietic factors. Instead, the possibility existed that the thymic microenvironment rendered pro–B cells unresponsive to B-lymphopoietic signals. To test this hypothesis, cells harvested from FTOC at various times after seeding lobes with bone marrow–derived CD45R+sIgM− cells were reseeded onto the S17 BM stromal cell line under B-cell permissive conditions.
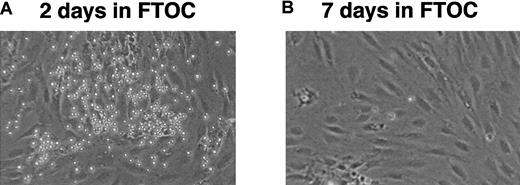
As shown in Table 1 and Figure3, cells harvested 2 days after culture in FTOC established vigorous long-term B-cell cultures when reseeded on S17 stroma. However, by 7 days of culture in FTOC, they were no longer able to do so. These results are consistent with data from 2 experiments demonstrating that CD45R+sIgM− cells directly isolated from the adult thymus did not establish long-term B-cell cultures following seeding on S17 stromal cells (data not shown).
Exposure to the thymic microenvironment renders pro–B cells unresponsive to B-lymphopoietic stimuli.
(A) Cells harvested from FTOCs 2 days after seeding lobes with 5 × 104 CD45R+sIgM− BM cells can still establish long-term cultures on S17 stroma. (B) CD45R+sIgM− cells can no longer establish long-term BM cultures following 7 days of culture in FTOCs. Original magnification, × 100.
Exposure to the thymic microenvironment renders pro–B cells unresponsive to B-lymphopoietic stimuli.
(A) Cells harvested from FTOCs 2 days after seeding lobes with 5 × 104 CD45R+sIgM− BM cells can still establish long-term cultures on S17 stroma. (B) CD45R+sIgM− cells can no longer establish long-term BM cultures following 7 days of culture in FTOCs. Original magnification, × 100.
Pro–B cells in the thymus are hyporesponsive to IL-7
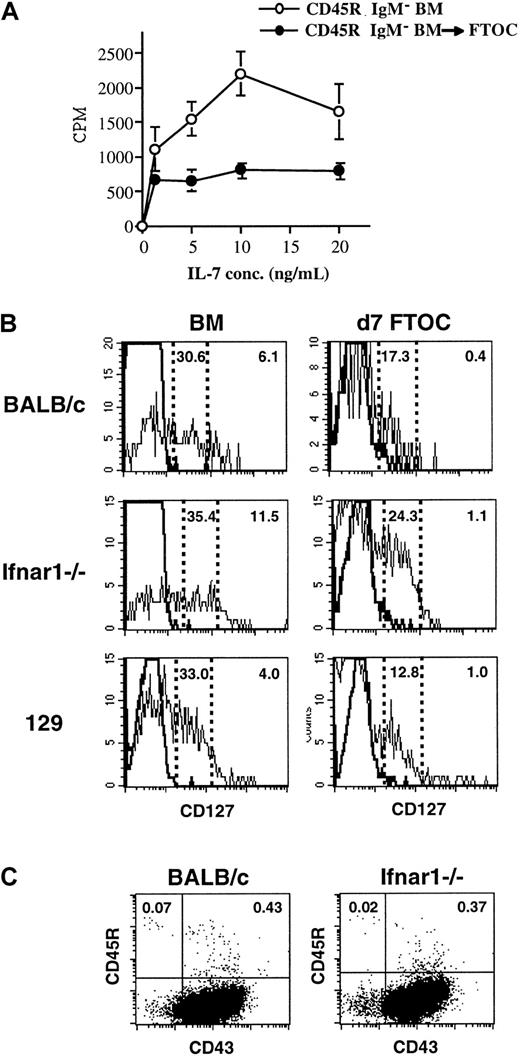
IL-7 is required for murine pro–B cells to proliferate and complete Ig heavy chain gene rearrangements and mature into pre–B cells.39 Because thymic pro–B cells accumulate in fraction C and cycle at a lower rate than their BM counterparts, their capacity to respond to IL-7 was examined. Following 7 days of culture in FTOC, cells were harvested from the lobes, and their proliferative response to IL-7 was compared to that of CD45R+sIgM− cells from fresh BM. The frequency of CD45R+CD43+ cells, which includes the most IL-7–responsive cells, in each population was determined by FACS. Based on this information, cultures were initiated using the same total number of CD45R+CD43+ cells from each source. As shown in Figure 4A, cells harvested from thymic lobes responded to IL-7, but the magnitude of their proliferative response was lower than that of the freshly harvested BM population.
IL-7 responsiveness and IL-7Ra receptor levels are decreased on B-lineage cells exposed to the thymic microenvironment.
(A) CD45R+sIgM− cells were cultured in FTOC, and 7 days later their proliferative response to increasing concentrations of IL-7 was compared to that of CD45R+sIgM− cells harvested from the BM. The frequency of CD45R+CD43+ cells in each population was determined by FACS, and based on this information, cultures were initiated using the same total number of CD45R+CD43+ cells from each source. (B) Seven days after seeding thymic lobes with 5 × 104CD45R+sIgM− BM cells, CD127 expression on CD45R+CD43+ pro–B cells was examined by FACS. Freshly isolated CD45R+CD43+ pro–B cells from the BM were examined in parallel. Studies were performed using cells from BALB/c (top), Ifnar1−/− (middle), and 129 strain mice. The frequency of high- and low-expressing CD127 cells is indicated in each plot. (C) Frequency of B-lineage cells in the thymus of BALB/c and Ifnar1−/−mice.
IL-7 responsiveness and IL-7Ra receptor levels are decreased on B-lineage cells exposed to the thymic microenvironment.
(A) CD45R+sIgM− cells were cultured in FTOC, and 7 days later their proliferative response to increasing concentrations of IL-7 was compared to that of CD45R+sIgM− cells harvested from the BM. The frequency of CD45R+CD43+ cells in each population was determined by FACS, and based on this information, cultures were initiated using the same total number of CD45R+CD43+ cells from each source. (B) Seven days after seeding thymic lobes with 5 × 104CD45R+sIgM− BM cells, CD127 expression on CD45R+CD43+ pro–B cells was examined by FACS. Freshly isolated CD45R+CD43+ pro–B cells from the BM were examined in parallel. Studies were performed using cells from BALB/c (top), Ifnar1−/− (middle), and 129 strain mice. The frequency of high- and low-expressing CD127 cells is indicated in each plot. (C) Frequency of B-lineage cells in the thymus of BALB/c and Ifnar1−/−mice.
This observation prompted an analysis of IL-7 receptor α chain (IL-7Rα [CD127]) expression on thymic B-lineage cells. Cells were harvested from thymic lobes 7 days after seeding with CD45R+sIgM− cells, and the level of CD127 expression on CD45R+CD43+ pro–B cells was compared to that on freshly isolated CD45R+CD43+ BM cells. The results indicated that the frequency of pro–B cells that express the IL-7Rα chain and those that express it at relatively high levels are reduced following 7 days of culture in FTOC (Figure 4B, top).
Thymic stromal cell–derived factors inhibit B lymphopoiesis
The above observations indicate that the thymic microenvironment is a source of mediators that render pro–B cells hyporesponsive to IL-7. To determine whether such inhibitors were soluble molecules, initial experiments used a modification of the myeloid to lymphoid long-term BM switch culture system29 to test whether thymic stromal cells could inhibit B-cell development in the absence of direct cell contact.
Within a week following transfer of long-term BM cultures established under the myeloid conditions described by Dexter and colleagues28 to B-lymphoid–permissive conditions,30 pro–B cells emerge, and by 3 weeks B-lineage cells predominate in the cultures. As shown in Figure5A, heterogeneous populations of bone marrow or thymic stroma growing in transwells were introduced into these cultures. By 3 weeks following transfer of long-term myeloid BM cultures to B-lymphoid permissive conditions, vigorous cultures containing CD45R+ cells were established (Figure5B). This same pattern of growth occurred when empty transwells (data not shown) or transwells containing heterogeneous BM stroma (Figure 5B) were placed in the cultures at the time of their transfer to the lymphoid conditions. However, when transwells containing heterogeneous preparations of thymic stromal cells were introduced into the cultures, CD45R+ cell production was dramatically reduced (Figure 5B), indicating that soluble mediators produced by the thymic microenvironment can inhibit intrathymic B lymphopoiesis.
Inhibitory signals from thymic stromal cells can counteract B lymphopoietic stimuli.
(A) The experimental protocol used to introduce transwells containing heterogeneous BM or thymic stromal cells into myeloid (Dexter) long-term BM cultures at the time of their transfer to B-lymphoid permissive conditions. (B) Nonadherent cells were harvested weekly from the cultures and analyzed for expression of CD45R. Data are representative of 3 experiments.
Inhibitory signals from thymic stromal cells can counteract B lymphopoietic stimuli.
(A) The experimental protocol used to introduce transwells containing heterogeneous BM or thymic stromal cells into myeloid (Dexter) long-term BM cultures at the time of their transfer to B-lymphoid permissive conditions. (B) Nonadherent cells were harvested weekly from the cultures and analyzed for expression of CD45R. Data are representative of 3 experiments.
Down-regulation of IL-7Rα occurs through a type 1 IFN receptor–independent mechanism
Type 1 interferons (IFNs) have been shown to inhibit the response of B-lineage cells to IL-7.40-43 In view of the above data indicating the involvement of soluble factors in limiting intrathymic B lymphopoiesis, it was of interest to assess the potential involvement of type 1 IFNs. To do so, CD45R+sIgM−cells from type 1 IFN receptor–deficient (Ifnar1−/−) mice were used to seed fetal thymic lobes. CD45R+sIgM− cells from 129 mice, the background strain of the Ifnar1−/−mice, were assayed in parallel. CD127 expression on CD45R+CD43+ cells harvested from these FTOCs was compared to that on bone marrow pro–B cells 7 days later. As shown in Figure 4B, exposure to the thymic microenvironment resulted in a greater than 90% reduction of CD127 high-expressing cells in Ifnar1−/− mice. This level of inhibition was comparable to that in BALB/c and greater than that in 129 strain mice. A prediction based on this observation is that the frequency of B-lineage cells in the thymus of Ifnar1−/− mice should not be elevated. This is in fact the case, as the frequency of B-lineage cells in the thymus of Ifnar1−/− mice was comparable to that in control animals (Figure 4C).
Resumption of B-cell maturation in the presence of exogenous IL-7
Taken together, the above data suggest that the growth and development of pro–B cells in the thymus is limited through IL-7Rα down-regulation. This event may in turn limit pro–B cell capacity to compete with T-cell progenitors for the IL-7 produced by the thymic microenvironment. A prediction based on this hypothesis is that increasing intrathymic IL-7 levels may in turn allow B cell growth. To test this premise, FTOCs were initiated with CD45R+sIgM− BM cells in the presence of 50 U/mL of exogenous IL-7. As shown in Figure6A, approximately half of the CD45R+ cells no longer expressed CD43 after 7 days of culture, suggesting that they had matured to the pre–B-cell stage. This result contrasts with the finding that the majority of B-lineage cells in non–IL-7 supplemented FTOC were CD45R+CD43+. In addition, although B-lineage cells harvested from day-7 FTOC were not able to establish long-term BM cultures on S17 BM stromal cells (Figure 3), they were able to do so if the medium was supplemented with IL-7 (Figure 6B). These cultures could be maintained for at least 3 weeks, and phenotypic analysis confirmed that the cultures contained B-lineage cells (data not shown).
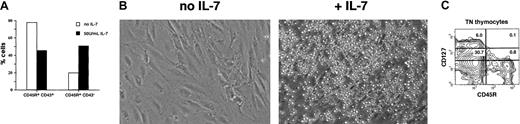
Responsiveness of thymic B cells to exogenous IL-7.
(A) Phenotype of cells harvested 7 days after seeding FTOC with 5 × 104 CD45R+sIgM− BM cells in the presence or absence of 50 U/mL of IL-7. (B) Cells harvested 7 days after seeding FTOC with CD45R+sIgM− BM cells could establish long-term cultures when seeded on S17 BM stromal cells in the presence of IL-7. Original magnification, × 100. (C) Expression of CD127 and CD45R on CD3−CD4−CD8− TN thymocytes harvested from the fresh thymus.
Responsiveness of thymic B cells to exogenous IL-7.
(A) Phenotype of cells harvested 7 days after seeding FTOC with 5 × 104 CD45R+sIgM− BM cells in the presence or absence of 50 U/mL of IL-7. (B) Cells harvested 7 days after seeding FTOC with CD45R+sIgM− BM cells could establish long-term cultures when seeded on S17 BM stromal cells in the presence of IL-7. Original magnification, × 100. (C) Expression of CD127 and CD45R on CD3−CD4−CD8− TN thymocytes harvested from the fresh thymus.
Since IL-7 is required for T-cell development,44 it was important to determine how levels of IL7Rα expression on thymic B- and T-cell progenitors compared. As shown in Figure 6C, the CD3−CD4−CD8− TN fraction of cells was isolated from the fresh thymus and analyzed for expression of CD127 and CD45R. This allowed levels of CD127 on CD45R+B-lineage cells to be compared to that on CD45R− cells, which primarily include T-cell progenitors. The data demonstrate that the frequency of cells that express CD127 is higher in the CD45R− TN thymocyte population and that they express it at higher levels than is found among CD45R+ cells.
Discussion
Both BM and thymic stromal cells express ligands required for direct interactions with developing B-lineage cells and secrete cytokines required for B-cell development. Despite these similarities, B-cell development in the thymus is limited. Studies aimed at investigating this phenomenon revealed that B-cell development is blocked at a relatively early stage of development in the thymus because signals produced by the thymic microenvironment inhibit that process.
Initial analyses focused on characterizing B-lineage cells harvested from the thymus. These studies demonstrated that the frequency of pro–B cells in cycle was lower in the thymus than in the BM and that B-lineage cells did not efficiently mature past the fraction C stage of development. When similar analyses were performed on bone marrow B-cell progenitors 7 days after being seeded in fetal thymic lobes in vitro, the same results were obtained. In addition to corroborating the results obtained with primary cells, this result established the fetal thymic organ culture system as a model for analyzing thymic B-cell production.
It is paradoxical that culture in the thymic microenvironment in the FTOC system inhibited B-cell development while other studies have reported the isolation of thymic stromal cell lines that can support B-cell differentiation. Indeed, our own laboratory characterized a thymic stromal cell line that efficiently supported the pre-B to B-cell transition.45 However, it is important to emphasize that the thymic stromal cell line in question only potentiated this latter phase of development but not the short- or long-term growth of pro–B cells. In fact, our characterization of numerous thymic stromal cell lines has so far failed to identify any capable of supporting long-term B-cell development. Furthermore, even if thymic stromal cells with such potential were isolated, they do not represent the thymic microenvironment as a whole. That is why, to assess the effects of the thymic microenvironment on B-cell development, primary cultures of heterogeneous thymic stromal cells rather than thymic stromal cell lines were used in the diffusion chamber studies.
That a mechanism to inhibit intrathymic B-cell development exists at all might seem puzzling, since the overwhelming majority of lymphoid cells in the thymus are T-lineage cells. Instead, the inefficient expansion and maturation of thymic pro–B cells could result from their failure to compete effectively for microenvironmental niches. However, it is important to focus this discussion on the most immature thymocytes contained within the TN compartment. Although most cells within this population are likely committed to the T lineage, it has been reported that 10% to 13% are CD45R+.13 If even a fraction of these cells did come into contact with thymic stromal cells that supported their growth and development, this in turn could compromise overall levels of T-cell production, particularly if enough mature B cells that affected the process of thymic education subsequently developed. Thus, it is logical to propose the existence of a mechanism(s) to specifically inhibit(s) thymic pro–B-cell development.
That such signals exist is supported by recent studies in which selective inactivation of Notch1 by gene targeting was performed. Radtke et al11 and Wilson et al12demonstrated that T-cell development was effectively blocked in mice in which Notch1 was conditionally inactivated and that there was an expansion in the number of B-lineage cells in the thymus. However, while the incidence of B-lineage cells was higher than in control mice, a critical point is that the total number of B-lineage cells in the thymus of these mice increased to only about 4 million cells. This number is considerably lower than the 100 million thymocytes that are present in the thymus of young animals. More recently, Han et al46 demonstrated that mice in which the RBP-J transcription factor, which associates with the intracellular domain of Notch to allow DNA binding, has been inactivated also exhibit impaired T-cell development and increased intrathymic B lymphopoiesis. However, even though Notch signaling in these mice was effectively blocked, the total number of thymic B-lineage cells was again only about 4 × 106 cells. Taken together, these results indicate that even when not competing for environmental niches with developing T cells, B-cell precursors do not undergo extensive expansion in the thymic microenvironment. Importantly, no differences between bone marrow B-lineage cells in mice in which Notch1 expression was conditionally inactivated and their control littermates were reported.
A recent study of mice that expressed a lunatic fringe transgene, which results in the inhibition of Notch1 activation, showed that their thymus contained up to 50 million B-lineage cells.13 That report would also seem to contradict the conclusion that the thymus actively inhibits B lymphopoiesis. However, while the results of that study could be interpreted to infer that inhibitors of thymic B lymphopoiesis do not exist, that conclusion may be too simplistic in view of the minimal level of B lymphopoiesis in the Notch1−/− and RBP-J−/− mice. Instead, it is important to consider that lunatic fringe normally functions as an intracellular mediator, but its transgenic expression resulted in secretion of the protein. This fact, combined with findings that the lunatic fringe protein can have effects beyond the inhibition of Notch1 activity,47 raises the distinct possibility that normal thymus physiology was altered in lunatic fringe transgenic mice. That this in turn impacted upon one or more regulatory pathways, such as those that inhibit B-cell development, must be considered.
In any case, the studies presented herein clearly demonstrate that exposure to the thymic microenvironment renders B-cell precursors unresponsive to B-lymphopoietic stimuli. After 7 days in thymic lobes, B-lineage cells could no longer proliferate and differentiate on BM stromal cells. Further analysis showed that this occurs because pro–B cells that have been exposed to the thymic microenvironment are hyporesponsive to IL-7. This seems to occur through IL-7Rα chain down-regulation. Proposing that interference with IL-7 signaling pathways is ultimately responsible for the inhibition of intrathymic B-cell development is entirely consistent with the observation that thymic pro–B cells or pro–B cells exposed to the thymic environment do not efficiently mature from the fraction C to the C′ stage of development. As described by Hardy et al,34 fraction C cells have undergone Ig heavy chain gene rearrangements at the D-J loci, but V-DJ rearrangements have not yet occurred. IL-7 is required for cells to complete the latter stage of Ig gene rearrangement and to transition from fraction C to the pre-B (fraction D) stage of development.
The long-term BM culture experiments described in this study suggest that soluble factors produced by thymic stromal cell(s) are at least partially responsible for the inhibition of thymic B-cell production. In fact, thymic stromal cells present in diffusion chambers were able to inhibit the emergence of B-lineage cells, even when precursors were in contact with a supporting layer of BM stromal cells. While aspecific effects of thymic stromal cells on B lymphopoiesis in this system through excessive consumption of nutrients cannot be excluded, it does not seem likely. First, there is no a priori reason to assume that confluent thymic and BM stroma differ significantly in the nutrients they consume. Second, cultures were fed twice weekly, and neither pH fluctuations nor excessive cell death were observed in cultures in which thymic stroma were present. Therefore, it is logical to propose that soluble factors from the thymic stroma are able to inhibit B-cell development and that their effects are potent enough to counteract positively acting B-lymphopoietic signals.
It has been reported that type 1 IFNs can inhibit the response of B-cell progenitors to IL-7.40-43 Because IFNs are produced in the thymus, it was of interest to determine if they were responsible for the observed effects by seeding pro–B cells from type 1 IFN receptor–deficient mice into fetal thymic lobes. This analysis revealed that the frequency of CD45R+CD43+cells that expressed CD127 at high levels had decreased by more than 90% after 7 days in FTOC. This finding, combined with the fact that the frequency of B-lineage cells in the thymus of these knockout mice was not elevated, strongly suggests that signaling through the type 1 IFN receptor is not responsible for the inhibition of intrathymic B lymphopoiesis. These findings would also seem to exclude the involvement of a new type 1 IFN family member, limitin, which has been proposed as a selective inhibitor of B-cell production.48
Taken together, the data in this and other studies make it possible to formulate a model in which checkpoints operative at multiple levels act in concert to limit B-cell development in the thymus. Initially, intrathymic lymphoid precursors, such as the CLP, might receive signals that preferentially potentiate their development along the T- rather than the B-cell lineage, and Notch1-activated signaling pathways may be critical at this juncture.10,11,49 Nevertheless, some pro–B cells develop, and their growth and differentiation is severely limited by thymic microenvironmental signals that render them hyporesponsive to IL-7. While IL-7 is not critical during human B lymphopoiesis,50 similar or alternative thymic stromal cell factors can be postulated to limit pro–B-cell expansion in the human thymus. The inhibition of intrathymic B lymphopoiesis may not be absolute, however, and some pro–B cells may mature into pre-B cells from which B lymphocytes may be generated. A few of these may be retained in the thymus as antigen-presenting cells,26while others are exported to the periphery.19
It is becoming increasingly appreciated that negative regulators play an important role in the regulation of hematopoiesis. In this regard, numerous mediators have been described that specifically inhibit B-cell production.51 However, these factors have generally been considered in terms of their effects on bone marrow B lymphopoiesis. The findings herein describe an unexpected role of negative regulatory factors in the homeostasis of thymic lymphocyte production. These data contribute to the understanding of how an organ that has the potential to support B-cell production limits that process and suggest that further comparisons of BM and thymic lymphopoiesis will provide additional insights into the regulation of primary lymphocyte production.
The authors appreciate the helpful discussions with Drs Ellen Rothenberg, Max Cooper, David Rawlings, and Andrew Farr.
Supported by National Institutes of Health grant HL60658.
Submitted March 7, 2002; accepted June 25, 2002. Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-03-0733.
Y.H. and E.M.-R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth Dorshkind, Department of Pathology and Laboratory Medicine and the Jonsson Comprehensive Cancer Center 173216, 10833 Le Conte Ave, Los Angeles, CA 90095; e-mail:kdorshki@mednet.ucla.edu.