Cytomegalovirus (CMV) infection causes significant morbidity and mortality in the setting of immunodeficiency, including the immune reconstitution phase following allogeneic stem cell transplantation (SCT). We assessed CMV-specific CD4+ and CD8+T-cell responses in 87 HLA-A*0201–positive (A2+) and/or B*0702-positive (B7+) allogeneic stem cell transplant recipients using HLA-peptide tetramer staining and cytokine flow cytometry (CFC) to examine the association of CMV-specific immune reconstitution and CMV antigenemia following SCT. Strong CMV-specific T-cell responses recovered in most subjects (77 of 87, 88%) after SCT. Frequencies of CMV-specific CD8+ T cells were significantly higher in those subjects who experienced early antigenemia relative to those who did not (2.2% vs 0.33%, P = .0002), as were frequencies of CMV-specific CD4+ T cells (1.71% vs 0.75%,P = .002). Frequencies of CMV-specific CD8+ T cells were also higher in subjects experiencing late antigenemia (2.4% vs 0.57%). When we combined tetramer staining and an assessment of cytokine production in a single assay, we found that individuals who experienced CMV antigenemia had lower tumor necrosis factor-α (TNF-α)–producing fractions of tetramer-staining CMV-specific CD8+ T cells than subjects who did not (25% vs 65%,P = .015). Furthermore, individuals at high risk for CMV reactivation, including patients with acute graft-versus-host disease and those receiving steroids, had low fractions of cytokine-producing CMV-specific CD8+ T cells (25% and 27%, respectively). These data suggest that the inability to control CMV reactivation following allogeneic SCT is due to the impaired function of antigen-specific CD8+ T cells rather than an inability to recover sufficient numbers of CMV-specific T cells.
Introduction
The cytotoxic T-cell response to chronic viral pathogens, including the β-herpesvirus cytomegalovirus (CMV), is marked by 4 phases: (1) activation and expansion of CD8+antigen-specific clones; (2) reduction of viral load through effector mechanisms, including cytolysis; (3) contraction, by apoptosis, of effector populations; and (4) maintenance of long-lived memory cells (reviewed by Ahmed and Gray1). Following allogeneic stem cell transplantation (SCT), CMV causes interstitial pneumonitis (IP), gastroenteritis, and a systemic wasting syndrome, and CMV seropositivity is an independent risk factor for adverse outcomes.2,3 Walter et al4 expanded and infused CMV-specific CD8+ clones in a dose-escalated fashion in allogeneic SCT recipients. Functional CMV-specific CD8+ T-cell responses were detected in all patients following infusion, although the magnitude of these responses declined in those who lacked detectable CD4+ CMV-specific T-cell responses.4 No patient who received these infusions went on to develop CMV disease, demonstrating the importance of functional CD8+ T cells in the control of CMV reactivation after SCT.4
The introduction of tetrameric HLA-peptide complexes5,6has dramatically altered our understanding of cytotoxic T-cell responses, including those specific for viruses7-11 and cancers.12,13 While HLA-peptide tetramers provide a useful measure of the frequency of antigen-specific CD8+ T cells, it is important to recognize that not all antigen-specific cells identified by tetramer staining are necessarily functional. Murine studies have revealed that the absence of antigen-specific CD4+ T cells may lead either to the deletion of antigen-specific CD8+ T cells or to the persistence of tetramer-staining CD8+ T cells that lack function in vivo.14 It has also been demonstrated that melanoma-specific CD8+ T cells may be less functional than Epstein-Barr virus (EBV)–specific CD8+ T cells in the same individual, in association with an ineffectual T-cell response against cancer.13 Appay et al15 examined CMV-specific and HIV-specific CD8+ T cells in human subjects coinfected with both viruses using a novel technique combining HLA-peptide tetramer staining and cytokine flow cytometry following epitopic peptide stimulation. This study demonstrated that a greater proportion of CMV-specific CD8+ T cells were functional relative to HIV-specific CD8+ T cells measured in the same subjects. Kostense et al16 have recently suggested that a decline in the function, rather than absolute numbers of HIV-specific CD8+T cells, was associated with progression to AIDS using similar methods.
Two previous studies have examined CMV-specific CD8+ T-cell recovery after SCT using HLA-peptide tetramers. Cwynarski et al17 studied 24 recipients of allogeneic SCT and found that recovery of CMV-specific cytotoxic T lymphocytes (CTLs) to levels above 10 × 106/L was associated with protection from CMV disease. Gratama et al18 also used HLA-peptide tetramers to examine 18 patients at risk for CMV reactivation following partially T-depleted SCT and concluded that tetramer-based measurement of CMV-specific CD8+ T-cell recovery might predict risk of progressive CMV infection. Neither study examined the association of CMV antigenemia to the magnitude of the CD4+ CMV-specific T-cell response. Furthermore, neither study assessed the functionality of CMV-specific CD8+ T cells either via cytokine flow cytometry (CFC) or using traditional cytotoxicity assays. No study to date has used both tetramer staining combined with functional CFC assessment to examine post-SCT immune recovery, as has been done for HIV-infected subjects.15 16
The development of new clinical transplantation strategies, including nonmyeloablative SCT,19 is increasing the number of potential SCT candidates. While nonmyeloablative regimens reduce SCT toxicity, infection and graft-versus-host disease (GVHD) remain significant challenges. To avoid GVHD, posttransplantation immunosuppressive therapy is required or stem cell grafts are depleted of GVHD-mediating T cells.20 Unfortunately, generalized T-cell depletion increases the risk of both infection2 and relapse21 due to the presumed loss of both viral- and cancer-specific effectors. Posttransplantation immunodeficiency needs to be addressed by adoptive immunotherapy or immunization strategies to hasten the recovery of T-cell responses against viral4,22and cancer antigens.12 The optimal design of such strategies, as well as those used to manage graft-versus-host disease and infection following transplantation, will depend on a clear understanding of quantitative and functional immune reconstitution following SCT. To better define essential elements of protective immunity to CMV, we used HLA-peptide tetramers and cytokine flow cytometry to study the relationship of quantitative and functional CD4+ and CD8+ T-cell responses to CMV-associated clinical events following SCT.
Patients and methods
Patient selection
Allogeneic SCT recipients at the University of Texas MD Anderson Cancer Center gave informed consent for a study of post-SCT immune function approved by the institutional review board. All subjects who received SCT between January 1999 and September 2001, who expressed HLA-A*0201 (A2) and/or HLA-B*0702 (B7) and had a defined risk factor for the development of CMV-associated complications (ie, donor and/or recipient CMV seropositivity), were considered eligible. Subjects were further selected based on the availability of cryopreserved peripheral blood mononuclear cells (PBMCs) collected approximately 3 months following SCT. Subjects were excluded in analyses of late antigenemia if they had follow-up shorter than to post-SCT day +150 and were censored at the time of death or relapse requiring cytotoxic chemotherapy. Investigators were blinded with respect to clinical status until quantitation of CMV-specific CD8+ T cells was completed.
Diagnosis and clinical management of CMV reactivation
CMV antigenemia was routinely monitored twice weekly through day 100 and then as clinically indicated. CMV pp65 antigenemia was assessed as previously described23 and considered positive based on at least 1 pp65+ cell per 106 neutrophils assessed. Early CMV antigenemia was defined as that occurring within the first 100 days following SCT, while CMV reactivation occurring following post-SCT day +100 was defined as late antigenemia. Recipients of matched unrelated donor and mismatched allografts received ganciclovir (GCV) prophylaxis during pre-SCT conditioning (until day −2) and post-SCT immune globulin (200 mg/kg intravenously weekly until day +100); patients with matched sibling donors received no routine CMV prophylaxis. Antigenemia was treated preemptively with GCV (5 mg/kg intravenously twice daily until clearance and then daily for 14 days) or with foscarnet in the event of GCV toxicity. All subjects received herpesvirus prophylaxis with valaciclovir (500 mg orally daily if not receiving GCV), a regimen with limited anti-CMV activity.24
Blood sampling
PBMCs were obtained by Ficoll gradient centrifugation from heparinized blood. For tetramer analysis, thawed PBMCs were resuspended in RPMI media with 10% FBS. Viability was assessed and generally exceeded 90%. Aliquots of 106 cells were used for HLA-peptide tetramer staining.
Analysis of CD8+ T-cell responses by HLA-tetramer staining
A2- and B7-restricted tetramers were produced using a method similar to that of Altman et al7 and as previously described.10,11 Briefly, complexes of HLA class I heavy chains, β2-microglobulin, and epitopic peptides were biotinylated. The resulting HLA-peptide complexes were recovered by fast protein liquid chromatography (FPLC) purification and ion exchange chromatography and made multivalent by incubation with streptavidin-phycoerythrin (PE; Sigma, St Louis, MO) at a molar ratio of 4:1. Tetramers used in this study were either HLA-A2–restricted (NLVPMVATV, amino acids 495-503; hereafter referred to as A2-pp65) or B7-restricted (TPRVTGGGAM, amino acids 417-26; B7-pp65) peptide epitopes of the CMV tegument protein pp65.11,25 26
To determine the frequency of tetramer-binding CD8+ T cells, PBMCs were incubated with PE-labeled tetramers in PBS with 2% fetal bovine serum (FBS) for 20 minutes at 37°C, followed by an additional 30 minutes with fluorescein isothiocyanate (FITC) anti-CD57 (Caltag, Burlingame, CA) and peridinin chlorophyll protein (PerCP) anti-CD8 antibodies (BD Biosciences, San Jose, CA). Cells were then fixed in 1% paraformaldehyde (PFA) and analyzed on a FACScan flow cytometer with data presented using CellQuest (BD Biosciences) and Flow Jo software (Tree Star, San Carlos, CA). Tetramer specificity was confirmed using CMV-specific CTL lines as positive controls; negative controls included PBMCs from CMV-seronegative and A2- and B7-negative individuals (data not shown). Absolute numbers of tetramer-staining cells were quantitated using total lymphocyte counts from clinical laboratory analysis and additional flow cytometric quantitation of CD8+ T-cell frequencies. The absolute number of tetramer-staining cells was calculated as the product of the following: (lymphocyte count)(frequency of CD8+ cells in the lymphocyte gate) (frequency of tetramer-staining cells in the CD8+ T-cell subset). In individuals assessed with both A2- and B7-restricted tetramers, the dominant response was used for analysis. In most of these subjects, the B7-restricted response was significantly higher than the A2-restricted response.
Assessment of CD4+ CMV-specific T-cell responses
A mixture of 138 overlapping 15-mer peptides spanning the entire sequence of CMV pp65 (a kind gift from H. Maecker, BD Biosciences) was used to stimulate either fresh or cryopreserved PBMCs as previously described.27 CD4+ T-cell responses to CMV were then determined using cytokine flow cytometry as previously described.27-29
Determination of functional responses of tetramer-staining cells
Functional analysis of tetramer-staining CD8+T cells was performed as previously described.15 Briefly, PBMCs were thawed, stained with HLA-peptide A2-pp65 and/or B7-pp65 tetramers, and then stimulated with the relevant epitopic peptide at a concentration of 10 μg/mL. Brefeldin A (Sigma) was added to prevent cytokine export. Cells were then fixed and permeabilized (using FACSPerm and FACSLyse solutions, BD Biosciences) and stained with monoclonal antibodies specific for tumor necrosis factor-α (TNF-α)–FITC and CD69-PerCP (BD Biosciences). The functional cell fraction was determined by flow cytometry as the TNF-α–expressing lymphocyte subset within the tetramer-staining CD8+ population.
Statistical analyses
Statistical analyses were performed using JMP statistical software (SAS Institute, Cary, NC). Intergroup comparisons were performed using the Mann-Whitney U test (for univariate nonparametric group analyses). All P values were 2-tailed and considered significant if less than .05. Results were presented using Prism (GraphPad, San Diego, CA) and Illustrator (Adobe, Seattle, WA) software on Macintosh computers (Apple Computer, Cupertino, CA).
Results
Patient characteristics and incidence of CMV antigenemia
PBMCs were collected at a median of 94 days (± 12 days) from 87 allogeneic recipients of SCTs who were at risk for CMV reactivation based on established clinical risk factors.30 31 We examined medical and laboratory records to assess the incidence of post-SCT CMV antigenemia and disease. A total of 16 subjects evaluable for early antigenemia were excluded from analyses of late antigenemia due to inadequate follow-up (ie, to less than post-SCT day +150). Early CMV antigenemia occurred in 52 (60%) of 87 subjects while late antigenemia occurred in 29 (41%) of 71 evaluable subjects. A total of 23 (79%) of 29 patients with late antigenemia had prior early antigenemia. Characteristics of study subjects are summarized in Table1.
CMV-specific CD4+ and CD8+ T-cell responses may be measured by cytokine flow cytometry and HLA-peptide tetramer staining
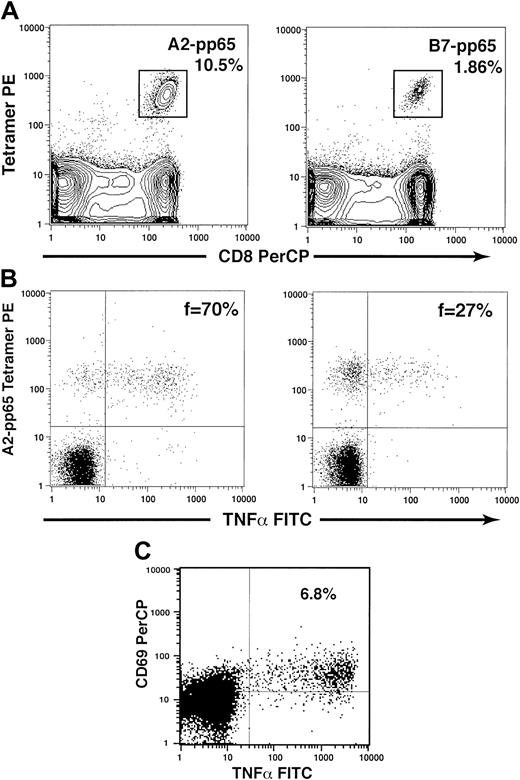
Flow cytometric methods were developed to assess CD4+and CD8+ T-cell responses to the CMV pp65 protein, the major viral gene product following productive infection and the primary target of protective CD8+ T-cell responses to CMV.25,32 Representative flow cytograms illustrating the methods used to characterize quantitative and functional CD4+ and CD8+ T-cell responses to CMV pp65 are shown in Figure 1. PBMCs were stained with HLA-pp65 tetramers and monoclonal antibodies specific for CD8 in all subjects. A2-pp65 and/or B7-pp65 tetramers were used in subjects expressing either HLA-A2 and/or B7 based on pre-SCT HLA typing (Figure1A). When sufficient cells were available, additional assays were performed to assess the fraction of tetramer-staining cells capable of functional activation after stimulation with cognate peptides and to assess the magnitude of the CD4+ T-cell response to the CMV pp65 protein. It has previously been shown that the fraction of tetramer-staining CD8+ T cells that is capable of responding functionally following stimulation may be assessed by combining these 2 approaches in one assay.15 We used a similar approach here to determine the relationship between the functional fraction of CMV-specific CD8+ T cells and CMV reactivation following SCT (Figure 1B). To evaluate functional CD4+ T-cell responses to CMV, PBMCs were stimulated with a mixture of 138 peptides, each 15 amino acids in length and overlapping by 11 amino acids to span the entire length of the CMV pp65 protein.27 Following stimulation, the fraction of CMV-specific CD4+ T cells was assessed by flow cytometry by determining the fraction of CD4+ T cells coexpressing CD69 and intracellular TNF-α as previously described (Figure 1C).
Quantitative and functional assessment of CMV-specific T-cell responses.
(A) Quantitative assessment of CMV-specific CD8+ T cells using HLA-peptide tetramers. HLA class I–peptide tetramers and monoclonal antibodies specific for CD8 were used to determine the frequency of CMV-specific CD8+ T cells specific for peptides derived from CMV pp65. Representative examples of staining using the A2-pp65 tetramer (10.5% of CD8+ T cells, left) and the B7-pp65 tetramer (1.86% of CD8+ T cells, right) are illustrated. (B) The function of CMV-specific CD8+ T cells may be assessed via a combination of tetramer staining and cytokine flow cytometry. Peripheral blood mononuclear cells were stained first with HLA-pp65 tetramers and then stimulated with the antigenic HLA-restricted peptide derived from pp65. The fraction of tetramer-staining cells producing intracellular cytokines was then determined by flow cytometry using antibodies specific for intracellular TNF-α. Representative examples of this analysis are shown for 2 subjects, who had relatively higher (70%, left) or lower (27%, right) functional fractions of tetramer-staining cells. (C) The proportion of CD4+ T cells responding to the CMV pp65 protein was assessed using cytokine flow cytometry following stimulation with a mixture of 138 overlapping peptides spanning the entire pp65 protein. CMV-specific CD4+ T cells were determined by the fraction of cells that were CD69+ that also produced intracellular TNF-α following stimulation. A representative example is shown for an individual with 6.8% of CD4+ T cells specific for CMV pp65.
Quantitative and functional assessment of CMV-specific T-cell responses.
(A) Quantitative assessment of CMV-specific CD8+ T cells using HLA-peptide tetramers. HLA class I–peptide tetramers and monoclonal antibodies specific for CD8 were used to determine the frequency of CMV-specific CD8+ T cells specific for peptides derived from CMV pp65. Representative examples of staining using the A2-pp65 tetramer (10.5% of CD8+ T cells, left) and the B7-pp65 tetramer (1.86% of CD8+ T cells, right) are illustrated. (B) The function of CMV-specific CD8+ T cells may be assessed via a combination of tetramer staining and cytokine flow cytometry. Peripheral blood mononuclear cells were stained first with HLA-pp65 tetramers and then stimulated with the antigenic HLA-restricted peptide derived from pp65. The fraction of tetramer-staining cells producing intracellular cytokines was then determined by flow cytometry using antibodies specific for intracellular TNF-α. Representative examples of this analysis are shown for 2 subjects, who had relatively higher (70%, left) or lower (27%, right) functional fractions of tetramer-staining cells. (C) The proportion of CD4+ T cells responding to the CMV pp65 protein was assessed using cytokine flow cytometry following stimulation with a mixture of 138 overlapping peptides spanning the entire pp65 protein. CMV-specific CD4+ T cells were determined by the fraction of cells that were CD69+ that also produced intracellular TNF-α following stimulation. A representative example is shown for an individual with 6.8% of CD4+ T cells specific for CMV pp65.
High frequencies of CD8+ CMV-specific T cells are present following SCT
Significantly higher frequencies of CD8+ CMV-specific T cells were seen in SCT patients than in a previously studied cohort of HIV-1–infected subjects8 (data not shown) despite the use of conditioning therapy and immunosuppressive drugs in the former group. Indeed, many SCT subjects (18 of 87, 21%) had extremely high frequencies of the CMV-specific CD8+ T cells (ie, > 5%), and nearly all (77 of 87, 88%) had measurable responses (ie, > 0.05%). One (A2+B7−) subject had 40% of peripheral CD8+ T cells that stained with the A2-pp65 tetramer, the highest such response reported to date and similar to that previously shown in the setting of acute infectious mononucleosis.10
Higher frequencies of CMV-specific CD4+ and CD8+ T-cell responses are present in subjects experiencing CMV antigenemia
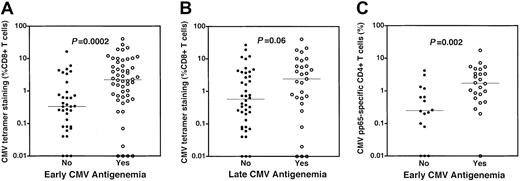
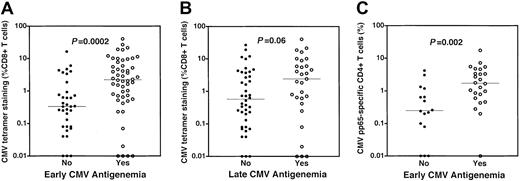
Dominant tetramer responses were stratified by the occurrence of early and late CMV antigenemia (Figure2A,B). Subjects who experienced early antigenemia (52 of 87 subjects) had higher frequencies (median 2.2% vs 0.33%, P = .0002, Figure 2A) of tetramer-staining CMV-specific CD8+ T cells. Absolute numbers of tetramer-staining CD8+ T cells were also higher in subjects experiencing early antigenemia (P = .0001, data not shown). Late CMV antigenemia occurred in 29 of 71 evaluable subjects. We examined the relationship between recovery of CMV-specific CD8+ T cells 3 months after SCT and the occurrence of late antigenemia. There was also a trend toward higher frequencies of CMV-specific CD8+ T cells in those subjects who experienced late antigenemia relative to those who did not (median 2.4% vs 0.57%, P = .06, Figure 2B). CD4+ T-cell responses specific for CMV were also significantly higher in those subjects experiencing early antigenemia (median 1.71% vs 0.75%,P = .002; Figure 2C). In 32 patients evaluable for late antigenemia in whom CD4+ CMV-specific T-cell responses were assessed, the median response was higher in those experiencing late antigenemia, although this was not statistically significant (median 2.01% vs 0.75%, data not shown). These data demonstrate that most individuals recover CD4+ and CD8+CMV-specific T-cell responses by 3 months following SCT and that CMV antigenemia is not associated with a simple quantitative deficiency of CMV-specific CD4+ or CD8+ T cells.
Higher frequencies of CMV-specific CD4+ and CD8+ T cells are present in subjects experiencing CMV reactivation after SCT.
(A) Higher frequencies of CMV-specific CD8+ T cells in patients with early CMV antigenemia. CMV-specific CD8+ T cells were measured by HLA-pp65 tetramer staining at a median of day 94 after SCT. Frequencies of CMV-specific CD8+ T cells were stratified by the occurrence of early antigenemia, traditionally defined by CMV reactivation prior to day 100 after SCT. Higher frequencies of CMV-specific CD8+ T cells were present in individuals who experienced early CMV antigenemia (○, median 2.2%) relative to those subjects who did not (●, median 0.33%) (P = .0002). (B) Frequencies of CMV-specific CD8+ T cells in patients stratified by the occurrence of late CMV antigenemia. A trend was noted toward higher frequencies of CMV-specific CD8+ T cells in individuals who experienced late CMV antigenemia (○, median 2.4%) relative to those subjects who did not (●, median 0.57%) (P = .06). (C) Higher frequencies of CMV-specific CD4+ T cells in patients with early CMV antigenemia. CD4+ T-cell responses were assessed using cytokine flow cytometry following stimulation of PBMCs using a mixture of overlapping peptides spanning the entire CMV pp65 protein sequence. The frequency of CMV-specific CD4+ T cells was determined by flow cytometry by assessing the simultaneous up-regulation of the CD69 activation marker and production of intracellular TNF-α in CD4+ T cells. Higher frequencies of CMV-specific CD4+ T cells were present in individuals who experienced early CMV antigenemia (○, median 1.71%) relative to those subjects who did not (●, median 0.75%) (P = .002). All P values attained by the Mann-Whitney test.
Higher frequencies of CMV-specific CD4+ and CD8+ T cells are present in subjects experiencing CMV reactivation after SCT.
(A) Higher frequencies of CMV-specific CD8+ T cells in patients with early CMV antigenemia. CMV-specific CD8+ T cells were measured by HLA-pp65 tetramer staining at a median of day 94 after SCT. Frequencies of CMV-specific CD8+ T cells were stratified by the occurrence of early antigenemia, traditionally defined by CMV reactivation prior to day 100 after SCT. Higher frequencies of CMV-specific CD8+ T cells were present in individuals who experienced early CMV antigenemia (○, median 2.2%) relative to those subjects who did not (●, median 0.33%) (P = .0002). (B) Frequencies of CMV-specific CD8+ T cells in patients stratified by the occurrence of late CMV antigenemia. A trend was noted toward higher frequencies of CMV-specific CD8+ T cells in individuals who experienced late CMV antigenemia (○, median 2.4%) relative to those subjects who did not (●, median 0.57%) (P = .06). (C) Higher frequencies of CMV-specific CD4+ T cells in patients with early CMV antigenemia. CD4+ T-cell responses were assessed using cytokine flow cytometry following stimulation of PBMCs using a mixture of overlapping peptides spanning the entire CMV pp65 protein sequence. The frequency of CMV-specific CD4+ T cells was determined by flow cytometry by assessing the simultaneous up-regulation of the CD69 activation marker and production of intracellular TNF-α in CD4+ T cells. Higher frequencies of CMV-specific CD4+ T cells were present in individuals who experienced early CMV antigenemia (○, median 1.71%) relative to those subjects who did not (●, median 0.75%) (P = .002). All P values attained by the Mann-Whitney test.
We chose to do a cross-sectional analysis at approximately 3 months after SCT (median, day +94) because this time point has traditionally served as an important milestone in the clinical management of CMV disease after transplantation and because lymphocyte recovery at this time point is sufficient to permit reliable analysis in most transplant recipients. Following the demonstration that CMV-specific CD4+ and CD8+ T-cell responses appeared to be more robust in those subjects who experienced viral reactivation either preceding or subsequent to our cross-sectional assessment, we conducted a subset analysis of those individuals who were assessed proximate to episodes of antigenemia. CMV-specific CD4+ and CD8+ T-cell responses by tetramer staining in this subset were statistically indistinguishable from those of other subjects who experienced early and/or late antigenemia (data not shown). In fact, the subject with the highest frequency of CMV-specific CD8+T cells by tetramer staining (40%) was assessed on the day CMV antigenemia was also detected. These data suggest that even in subjects assessed at or near the time of CMV reactivation, deficiencies in CMV-specific T cells were not evident.
CMV-specific CD8+ T cells measured by tetramer staining are less functional in individuals with CMV antigenemia
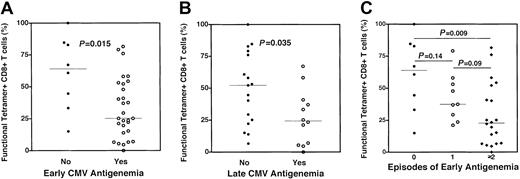
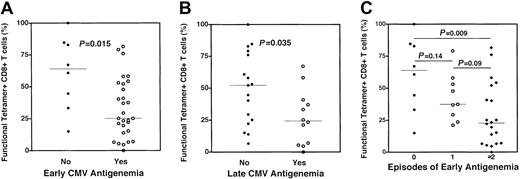
In murine models14 and in some patients with cancer,13 it has been demonstrated that tetramer-staining CD8+ T cells may persist but lack effector function. To assess the functionality of recovering CMV-specific CD8+ T cells, we combined tetramer staining with epitopic peptide stimulation and CFC as previously described.15 In this instance, function was assessed by the ability of a tetramer-stained CD8+ T cell to produce intracellular TNF-α following stimulation with its cognate peptide. Representative examples of 2 subjects who had relatively high (70%) and low (27%) functional fractions of CMV-specific CD8+ T cells are shown (Figure1B). In 37 subjects stratified by the occurrence of early CMV antigenemia, the functional fraction of CMV-specific CD8+ T cells was significantly lower in those subjects who experienced early antigenemia relative to those who did not (median 25% vs 65%,P = .015, Figure 3A). A similar analysis of individuals assessed for CMV-specific CD8+ T-cell function and stratified by the occurrence of late antigenemia revealed a lower cytokine-producing fraction of tetramer-stained CD8+ T cells in those subjects experiencing late antigenemia relative to those who did not (28% vs 53%, P = .035, Figure 3B). Further analysis of subjects stratified by the number of episodes of early antigenemia revealed a stepwise decrease in CD8+ CMV-specific T-cell function in subjects experiencing one (or ≥ 2) episode. In aggregate, these data suggest that the occurrence of CMV antigenemia following SCT is closely correlated with a decreased functional fraction of CMV-specific CD8+ T cells as assessed by cytokine production and not associated with decreased frequencies of antigen-specific CD8+ T cells measured by tetramer staining.
Dysfunctional CMV-specific CD8+ T cells are present in individuals experiencing CMV reactivation following SCT.
A combination of HLA-pp65 tetramer staining and cytokine flow cytometry was used to assess the functional fraction of CMV-specific CD8+ T cells stratified by the occurrence of CMV antigenemia. (A) Individuals experiencing early CMV antigenemia (ie, prior to post-SCT day +100) had a significantly lower fraction of tetramer-staining CMV-specific CD8+ T cells capable of cytokine production following cognate peptide stimulation (○, median 25%) relative to those who did not experience early CMV antigenemia (●, median 65%) (P = .015). (B) Individuals experiencing late CMV antigenemia (ie, after post-SCT day +100) had a significantly lower fraction of tetramer-staining CMV-specific CD8+ T cells capable of cytokine production following cognate peptide stimulation (○, median 28%) relative to those who did not experience early CMV antigenemia (●, median 53%) (P = .035). (C) CMV reactivation is associated with a decreased functional fraction of CMV-specific CD8+ T cells. Functional fractions of CMV-specific CD8+ T cells were stratified by the number of episodes of CMV reactivation occurring prior to day 100 after SCT. A strong trend toward a decreased functional fraction of CMV-specific CD8+ T cells was noted between groups of subjects stratified by the number of episodes of CMV antigenemia (● = none, median 64%; ○ = 1 episode, median 38%; ♦ = 2 or more episodes, median 23%). P values are noted for intergroup comparisons, and were attained by the Mann-Whitney test.
Dysfunctional CMV-specific CD8+ T cells are present in individuals experiencing CMV reactivation following SCT.
A combination of HLA-pp65 tetramer staining and cytokine flow cytometry was used to assess the functional fraction of CMV-specific CD8+ T cells stratified by the occurrence of CMV antigenemia. (A) Individuals experiencing early CMV antigenemia (ie, prior to post-SCT day +100) had a significantly lower fraction of tetramer-staining CMV-specific CD8+ T cells capable of cytokine production following cognate peptide stimulation (○, median 25%) relative to those who did not experience early CMV antigenemia (●, median 65%) (P = .015). (B) Individuals experiencing late CMV antigenemia (ie, after post-SCT day +100) had a significantly lower fraction of tetramer-staining CMV-specific CD8+ T cells capable of cytokine production following cognate peptide stimulation (○, median 28%) relative to those who did not experience early CMV antigenemia (●, median 53%) (P = .035). (C) CMV reactivation is associated with a decreased functional fraction of CMV-specific CD8+ T cells. Functional fractions of CMV-specific CD8+ T cells were stratified by the number of episodes of CMV reactivation occurring prior to day 100 after SCT. A strong trend toward a decreased functional fraction of CMV-specific CD8+ T cells was noted between groups of subjects stratified by the number of episodes of CMV antigenemia (● = none, median 64%; ○ = 1 episode, median 38%; ♦ = 2 or more episodes, median 23%). P values are noted for intergroup comparisons, and were attained by the Mann-Whitney test.
The magnitude of the CD4+ T-cell response to CMV is positively associated with the frequency of CD8+ T cells specific for CMV but not the functional fraction of tetramer-stained CD8+ T cells
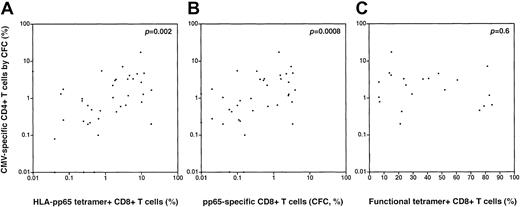
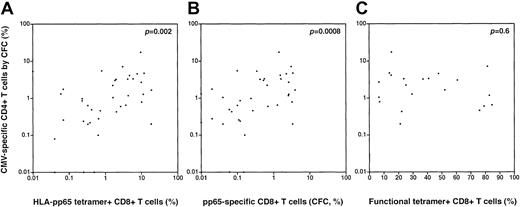
In subsets of patients analyzed using CFC to determine the frequency of CMV-specific CD4+ T cells specific for pp65, we conducted regression analyses to assess the association between the magnitude of the CD4+ pp65-specific T-cell response and CD8+ T-cell responses to CMV pp65 using tetramer staining and/or CFC. We found a positive association between the magnitude of the CD4+ CMV-specific T-cell response (by pp65 CFC) and both the frequency of HLA-pp65 tetramer-staining cells (p = .002, Spearman; Figure4A) and the frequency of functional CMV-specific CD8+ T cells by CFC following pp65 peptide stimulation (p = .0008, Spearman; Figure 4B). There was no significant correlation between the frequency of CMV-specific CD4+ T cells (by pp65 CFC) and the functional fraction of tetramer-staining cells (assessed by the fraction of TNF-α–producing cells following peptide stimulation) (p = .6; Figure 4C).
Correlations between the CD4+ T-cell response to CMV pp65 (by CFC) and measures of the CD8+ T-cell response to CMV.
(A) There is a significant positive correlation between the magnitude of the CD4+ pp65-specific T-cell response (by CFC) and the magnitude of the CMV-specific CD8+ T-cell response by HLA-peptide tetramer staining (p = .002, Spearman) (B) There is a significant positive correlation between the magnitude of the CD4+ pp65-specific T-cell response (by CFC) and the magnitude of the CMV-specific CD8+ T-cell response by CFC following pp65 peptide stimulation (p = .0008, Spearman). (C) There is no significant correlation between pp65-specific CD4+ T cells (by CFC) and the functional fraction of tetramer-staining CD8+ T cells (by assessment of TNF-α production in tetramer-positive cells following cognate peptide stimulation); p = .6, Spearman.
Correlations between the CD4+ T-cell response to CMV pp65 (by CFC) and measures of the CD8+ T-cell response to CMV.
(A) There is a significant positive correlation between the magnitude of the CD4+ pp65-specific T-cell response (by CFC) and the magnitude of the CMV-specific CD8+ T-cell response by HLA-peptide tetramer staining (p = .002, Spearman) (B) There is a significant positive correlation between the magnitude of the CD4+ pp65-specific T-cell response (by CFC) and the magnitude of the CMV-specific CD8+ T-cell response by CFC following pp65 peptide stimulation (p = .0008, Spearman). (C) There is no significant correlation between pp65-specific CD4+ T cells (by CFC) and the functional fraction of tetramer-staining CD8+ T cells (by assessment of TNF-α production in tetramer-positive cells following cognate peptide stimulation); p = .6, Spearman.
Lower functional fractions of CMV-specific CD8+ T cells are present in individuals at a higher risk for CMV reactivation
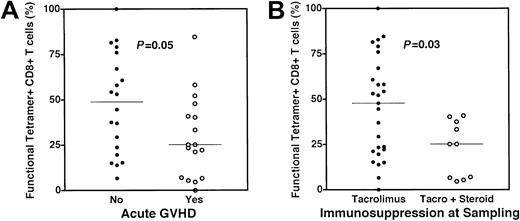
Following the observation that lower functional fractions of CMV-specific CD8+ T cells were present in those subjects experiencing antigenemia, we analyzed functional fractions of CMV-specific CD8+ T cells with respect to known risk factors for CMV reactivation. Lower functional fractions of CMV-specific CD8+ T cells, as assessed by intracellular TNF-α production, were present in those individuals treated with combined immunosuppressive therapy with tacrolimus and steroids (27% vs 48% for those receiving therapy with tacrolimus alone,P = .03, Figure 5A). Individuals who experienced acute GVHD also had a lower functional fraction of CMV-specific CD8+ T cells (25% vs 49% for those with no acute GVHD, P = .05, Figure 5B). For each of these comparisons, the frequencies of CMV-specific CD8+ T cells measured by tetramer staining alone were not significantly different between groups, although the lower cytokine-producing fraction correctly identified the group at higher risk for CMV reactivation in each case.
Association of acute graft versus host disease and steroid therapy with decreased functional fractions of CMV-specific CD8+ T cells.
(A) Functional fractions of CMV-specific CD8+ T cells were assessed using a combination of HLA-pp65 tetramer staining and cytokine flow cytometry as previously discussed. Individuals who experienced acute GVHD (○, median 25%) also had a lower functional fraction of CMV-specific CD8+ T cells (●, median 49% for those with no acute GVHD) (P = .05). (B) Dysfunction of CMV-specific CD8+ T cells in subjects treated with steroids. Individuals receiving tacrolimus alone at the time of sampling (●, median 48%) had a higher fraction of functional CMV-specific CD8+ T cells than those subjects receiving steroids in addition to tacrolimus (○, median 27%) (P = .03). All P values attained by the Mann-Whitney test.
Association of acute graft versus host disease and steroid therapy with decreased functional fractions of CMV-specific CD8+ T cells.
(A) Functional fractions of CMV-specific CD8+ T cells were assessed using a combination of HLA-pp65 tetramer staining and cytokine flow cytometry as previously discussed. Individuals who experienced acute GVHD (○, median 25%) also had a lower functional fraction of CMV-specific CD8+ T cells (●, median 49% for those with no acute GVHD) (P = .05). (B) Dysfunction of CMV-specific CD8+ T cells in subjects treated with steroids. Individuals receiving tacrolimus alone at the time of sampling (●, median 48%) had a higher fraction of functional CMV-specific CD8+ T cells than those subjects receiving steroids in addition to tacrolimus (○, median 27%) (P = .03). All P values attained by the Mann-Whitney test.
Discussion
CMV continues to cause significant morbidity and mortality in the setting of SCT.2,33 34 To better define the role of CMV-specific CD8+ T-cell recovery in CMV control, we conducted the largest analysis to date using HLA-peptide tetramers to study SCT recipients. Several important conclusions emerged from this study, including the following: (1) high frequencies of CMV-specific CD4+ and CD8+ T cells recover in most subjects following SCT; (2) risk of CMV antigenemia was closely associated with a lower functional fraction of CMV-specific CD8+ T cells as assessed by cytokine production and not a lower frequency or absolute number of CMV-specific CD4+ or CD8+ T cells; (3) lower cytokine-producing fractions of CMV-specific CD8+T cells were present in individuals known to be at high risk for CMV reactivation, including those with acute GVHD and those receiving steroids for immunosuppression. These findings have important implications for our understanding of protective immunity to CMV in general and for the design of SCT strategies in particular.
Prior studies have suggested that the use of routine GCV prophylaxis during the first 100 days after SCT, rather than preemptive therapy initiated by evidence of viral reactivation, leads to an increased incidence of late CMV antigenemia.35 It is widely believed that this phenomenon is caused by impairment of CMV-specific T-cell recovery due to GCV-induced suppression of viremia, with attendant decreases in priming of CMV-specific CD8+ T cells.35 In this study, we found that the numbers of CMV-specific CD4+ and CD8+ T cells were actually increased in those subjects who experienced early antigenemia, even though preemptive ganciclovir therapy was routinely used in those subjects to suppress viral replication. Thus, these data do not support a model wherein late antigenemia is due solely to the failure to recover significant numbers of CMV-specific CD4+ or CD8+ T cells by day 100 after SCT. Furthermore, our data are at odds with prior suggestions that the recovery of a sufficient number of CMV-specific CD8+ T cells determined by tetramer staining (eg, 10 CMV-specific CD8+ T cells per microliter17) may be used to identify those at low risk for further CMV reactivation after SCT.17 18 Indeed, some of the highest frequencies and absolute numbers of CMV-specific CD8+ T cells were noted in those subjects who experienced early and/or late CMV reactivation.
Our prior studies in HIV-1 infection demonstrated diminished CD4+ T-cell responses to CMV in subjects with either active or relapsing CMV retinitis.29,36 Studies of CD4+ knock-out mice have demonstrated that the lack of CD4+ T-cell help may result in the deletion of CD8+ T-cell responses, as measured by tetramer staining, or the persistence of specific T cells lacking effector function. In this study, we found a positive correlation between the frequency of CD4+ CMV-specific cells and the frequency of CD8+ CMV-specific T cells measured either by tetramer staining or pp65 CFC, consistent with results of our prior studies in HIV-infected subjects.8 However, we failed to demonstrate a positive correlation between the magnitude of the CD4+T-cell response by CFC and the functional fraction of tetramer-staining CD8+ T cells.
Several clinical and experimental limitations should be considered in interpreting our findings. The incidence of late CMV antigenemia may represent an underestimation of the true value. Subjects who were asymptomatic at that stage of transplantation may have presented for medical attention less frequently and may not have had routine assessment of CMV antigenemia as a result. The functional analyses we carried out were a surrogate for traditional cytotoxicity assays. Flow cytometry–based analyses have been shown to correlate closely with traditional measures of cytotoxicity and have several advantages.11 They do not require the presence of target cell lines individually derived from each donor, may be reliably performed using the extremely limited numbers of PBMCs usually available in lymphopenic SCT recipients, and do not require long periods of ex vivo culture that may alter the functional characteristics of responding CD8+ T cells.
While other studies have analyzed interferon-γ (IFN-γ) production, we chose TNF-α because of our extensive experience using this cytokine as an end point in CFC assays in CD4+ and CD8+ T cells.8,29,36 Nixon et al recently performed a detailed analysis of individual cytokines and cytotoxic granule constituents expressed in subsets of CMV-specific CD8+ T cells activated by cognate peptides.37Concomitant production of TNF-α and IFN-γ demarcated the dominant effector subset of CD8+ T cells, while coexpression of IL-2, TNF-α, and IFN-γ were noted in the next most common subset of CMV-activated CD8+ T cells.37 Picker et al previously established a similar pattern of TNF-α and IFN-γ coexpression in activated CMV-specific CD4+ T cells.28 In our prior studies of subjects infected with HIV-1, we found that impaired TNF-α production in CMV-specific T cells was associated with susceptibility to CMV end-organ disease, including retinitis.29 36 While these data suggest that the inability to produce TNF-α accurately reflects impaired T-cell function, future studies will be required to confirm that this phenotype correlates closely with dysfunction in other assays of effector activity (eg, lysis of targets in traditional cytotoxicity assays).
We chose to focus our analysis on the CMV pp65 protein, given its well-characterized role as an immunodominant target of the CD8+ T-cell response to CMV. It is important to recognize that other CMV proteins, including the immediate early 1 protein, may also serve as important targets of the CMV-specific immune response.38 Indeed, the contribution of pp65-specific responses to the overall immune response appears to be greater in the CD8+ T-cell response relative to the CD4+T-cell response to CMV.27 Assessing responses to the CMV pp65 protein also afforded the advantage of directly correlating the association between the pp65-specific CD4+ and CD8+ T-cell immune response (measured by tetramer staining and CFC) and in vivo antigen production, because pp65 antigenemia was the end point of the assay we used to detect CMV reactivation.
Our data suggest that the presence of dysfunctional CD8+ T cells specific for CMV, rather than insufficient numbers of CD4+ or CD8+ T cells, is most closely associated with viral reactivation following allogeneic SCT for malignancy. These findings are consistent with data from HIV-infected subjects demonstrating that HIV-specific CD8+ T cells may persist but lack function in those individuals.15,16 How is it that a lower functional fraction of CMV-specific CD8+T cells could decrease the ability of persistent functional CMV-specific cells to control CMV reactivation following SCT? It has recently been demonstrated that higher-affinity CD8+ T cells may effectively compete for class I–peptide complexes at the T cell–dendritic cell interface, providing a mechanism for affinity maturation of CD8+ T cells.39,40 It has been suggested that the mechanism for this competition may be the direct “stripping” of major histocompatibility complex (MHC)–peptide complexes from the dendritic cell surface, as previously demonstrated ex vivo.41 It is conceivable that anergized CMV-specific CD8+ T cells might still compete for these MHC-peptide complexes, limiting the access of functional T cells to antigenic stimuli, effectively blunting the protective immune response. While our experiments do not address such potential mechanisms, they do suggest further avenues for future study.
These findings have important consequences for the development of immunotherapeutic strategies for the management of CMV disease after SCT. The demonstration that most individuals experiencing CMV antigenemia after SCT have significant numbers of CMV-specific CD4+ and CD8+ T cells suggests that greater attention should be directed at the preservation of the function of such cells. For example, the use of tacrolimus for the prevention and/or therapy of GVHD resulted in the dysfunction of CMV-specific CD8+ T cells only following the addition of steroids. These data suggest that the increased incidence in CMV reactivation observed in individuals treated with steroids is more likely due to the dysfunction of CMV-specific CD8+ T cells than other mechanisms, such as apoptosis of effector populations,42because total numbers of CMV-specific CD8+ T cells were similar in those receiving steroids versus tacrolimus alone.
Because the observations in this report were derived from cross-sectional analysis, it will be important to confirm these findings in prospective studies analyzing the number and function of CMV-specific CD4+ and CD8+ T cells. Such studies may determine the utility of the functional methods used here in the assessment of optimal regimens used for the prophylaxis and therapy of GVHD following SCT as well as the impact of active interventions (eg, immunization or the adoptive transfer of antigen-specific T-cell populations) on the number and function of viral and cancer-specific T cells in recipients of allogeneic SCTs.
The authors thank the patients and bone marrow transplantation clinicians at the University of Texas MD Anderson Cancer Center for their contribution to these studies. We thank Eric Wieder, Jeff Harris, Doug Nixon, and Mike McCune for critical review of the manuscript and Rima Saliba for reviewing the statistical calculations.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-05-1387.
Supported by grant support from the Leukemia Lymphoma Society of America (Translational Research Program grant no. 6606-01 to K.V.K. and grant no. 6148-99 to J.J.M.) and the National Institutes of Health (RO1 CA81247 to J.J.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Krishna Komanduri, Section of Transplant Immunology FC5.2038, Department of Blood and Marrow Transplantation, MD Anderson Cancer Center (Box 448), 1515 Holcombe Blvd, Houston, TX 77030; e-mail: kkomandu@mail.mdanderson.org.