In the present study we analyzed the role of phophatidylinositol-3 kinase (PI-3K) in B chronic lymphocytic leukemia (B-CLL) cells. PI-3K is activated by many stimuli and is linked to several different signaling pathways. We demonstrated that inhibition of PI-3K by a specific inhibitor, LY294002, induced apoptosis in B-CLL cells in vitro. This effect was specific for the inhibition of PI-3K because inhibition of other signaling pathways such as extracellular signaling–regulated kinase (ERK), p38, or p70S6 kinase did not affect spontaneous apoptosis. Furthermore, PI-3K was constitutively activated in freshly isolated B-CLL cells. Corresponding to enhanced apoptosis, LY294002 down-regulated expression of the antiapoptotic proteins X-linked inhibitor of apoptosis protein (XIAP) and Mcl-1. Next, we investigated which factors downstream of PI-3K were activated in B-CLL cells. We demonstrated that protein kinase B/Akt is expressed in all tested CLL samples but no activation of Akt was detected. In contrast, we observed a constitutive activation of protein kinase Cδ (PKCδ) in freshly isolated B-CLL cells. PKCδ is linked to PI-3K and is phosphorylated at Thr505 in response to PI-3K activation. We further demonstrated that tyrosine phosphorylation and activity of PKCδ were dependent on PI-3K activity in B-CLL cells. Inhibition of PKCδ by the specific inhibitor Rottlerin strikingly enhanced apoptosis. In contrast, peripheral blood B cells of healthy donors were resistant to inhibition of PI-3K or PKCδ. We conclude that activated PI-3K might be important in the pathogenesis of B-CLL, and survival signals might be mediated via PKCδ. Therefore, inhibition of PI-3K or PKCδ may be an innovative approach to treat B-CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in the Western world. It is characterized by the accumulation of monoclonal CD5+ mature B cells, with a high percentage of cells arrested in the G0/G1phase of the cell cycle.1 A profound defect in programmed cell death as well as in cell cycle progression is a major step in the pathogenesis of B-cell CLL (B-CLL).2 Despite the development of new chemotherapeutic drugs such as purine nucleotides, the disease remains incurable and progression occurs in every patient after an unpredictable clinical course. A variety of chromosomal aberrations have been described such as 13q14 and 11q22 deletions and trisomy 12,3,4 but no general molecular defect has been found and little is known about the molecular pathogenesis of the disease. Apoptosis resistance has been associated with high levels of the antiapoptotic protein bcl-2 in many B-CLL samples.5 In addition, overexpression of the antiapoptotic protein Mcl-1 in B-CLL has been correlated with failure to achieve complete remission through chemotherapy.6 However, it is still unknown which factors are responsible for the disproportion of proapoptotic and antiapoptotic proteins in B-CLL.
There is evidence for the existence of constitutively activated signaling pathways in CLL, as Frank et al identified constitutive serine phosphorylation of signal transducer and activator of transcription 1 and 3 (STAT1 and STAT3).7 Furthermore, B-CLL cells contain high levels of nuclear factor-κB (NF-κB) activity,8 but involved upstream activators still need to be determined. The biologic meaning of constitutively activated signaling pathways in B-CLL is largely unknown.
Phosphatidylinositol-3 kinase (PI-3K) is an enzyme whose inositol lipid products are key mediators of several distinct intracellular pathways. PI-3K consists of a 110-kDa catalytic subunit and a tightly associated regulatory subunit p85.9 PI-3K is involved in several signaling transduction pathways in B cells such as CD40 signaling,10 BCR signaling,11 and signaling of a variety of cytokines. It has been shown that PI-3K activates the serine/threonine kinase Akt/protein kinase B (PKB).12 Akt binds to the products of PI-3K, phosphatidylinositol 3,4-bisphosphate (PI 3,4-P2), and phosphatidylinositol 3,4,5-trisphosphate (PI 3,4,5-P3). Then Akt becomes activated by phosphorylation at Thr308 and Ser473 through the action of the 3-phosphoinositide–dependent kinase PDK1.13 One major activity of Akt is to mediate cell survival in a broad spectrum of cells. Akt overexpression has been found in cancer cells of pancreatic, ovarian, or breast origin.14,15 Antiapoptotic signals mediated by Akt include phosphorylation of the bcl-2 counterpart bad, inhibition of caspase-9,16 and activation of NF-κB17 and p70S6 kinase.18 PI-3K signaling is also linked to novel and atypical protein kinase C (PKC) isoforms.19 PKC is a family of isoenzymes classified by their dependence on cofactors and may be regulated by several independent mechanisms. We investigated PKCδ expression and activation in B-CLL cells, because this enzyme is expressed in lymphocytes, activated in response to B-cell receptor ligation,20 and associates with PI-3K in response to cytokine stimulation of hematopoietic cells.21 PKCδ is involved in regulation of the cell cycle and the programmed cell death; overexpression of PKCδ causes cell cycle arrest in a variety of cell types, whereas cell cycle transition is not affected by overexpression of other PKC isoforms.22,23 PKCδ inhibits the expression of cyclin D1 and cyclin E accompanied with an up-regulation of p27 in vascular smooth muscle cells.24 PKCδ-deficient mice show autonomous hyperproliferation of B cells, suggesting that PKCδ negatively regulates B-cell proliferation.25 Other findings have shown that PKCδ also might play a role in apoptosis. In cells undergoing apoptosis, PKCδ is cleaved by caspase-3 and this cleaving fragment is sufficient to induce apoptosis itself.26 Because of its ambivalent effects on cell cycle progression and apoptosis, PKCδ might also be important in CLL.
In the present work we have shown for the first time that PI-3K is constitutively activated in B-CLL cells and that inhibition of PI-3K induces apoptosis in a way that is independent of the downstream kinase Akt. We further demonstrate that PKCδ is activated in freshly isolated B-CLL cells and that inhibition of this enzyme strikingly induces apoptosis in contrast to normal peripheral blood B cells. Our findings suggest that the PI-3 kinase/PKCδ pathway plays an important role in the defect of programmed cell death in B-CLL cells.
Materials and methods
Cell samples
After the patients gave informed consent according to the institutional review board at the Technical University of Munich, peripheral blood was obtained from patients with a diagnosis of B-CLL according to clinical and immunophenotypic criteria. Patients were either untreated or had not received chemotherapy or steroids for a period of at least 3 months prior to the investigation. At the time of analysis all patients were clinically stable, free from infectious complications, and undergoing routine clinical outpatient review. Control samples were obtained from peripheral blood of healthy donors.
Reagents and antibodies
Monoclonal antibodies (mAbs) specific for bcl-2, X-linked inhibitor of apoptosis protein (XIAP), and PKCδ were purchased from BD Transduction Laboratories (Heidelberg, Germany); mAbs against Akt, phospho473-Akt, phosphothreonine505-PKCδ were obtained from New England Biolabs (Schwalbach, Germany). Specific mAbs against caspase-3 and bax were from Pharmingen (San Diego, CA), and against β-actin from Sigma (Deisenhofen, Germany). The mAb specific for Mcl-1 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and for antiphosphotyrosine, clone 4G10, from Upstate Biotechnology (Lake Placid, NY). The protein kinase inhibitors LY294002, wortmannin, Rottlerin, SB 203580, PD 98059, and rapamycin were obtained from Calbiochem (Schwalbach, Germany) as well as the caspase inhibitor zvad.fmk and caspase-3 inhibitor z-devd-fmk.
Separation procedures
Peripheral blood mononuclear cells (PBMNCs) were isolated from heparinized blood samples by centrifugation over a Ficoll-Hypaque layer (Biochrom, Berlin, Germany) of 1.077 g/mL density. For separation of CLL B cells, PBMNCs were incubated with anti-CD2 and anti-CD14 magnetic beads (Dynabeads M450, Dynal Biotech, Oslo, Norway) according to the manufacturer's instructions. Such prepared B cells from patients with CLL were more than 98% pure as assessed by direct immunofluorescence using Coulter Epics XL (Coulter, Hamburg, Germany). For separation of peripheral blood B cells from healthy donors, PBMNCs were incubated with anti-CD19 magnetic beads and isolated B cells were released from CD19 beads by using CD19 DETACHaBEAD (Dynal Biotech) according to the manufacturer's instructions.
Culture conditions
Purified normal and leukemic B cells were cultured in RPMI 1640 medium (Biochrom) supplemented with 10% fetal calf serum (Biochrom), penicillin/streptomycin 50 IU/mL, sodium pyruvate 1 mM,l-glutamine 2 mM, l-asparagine 20 μg/mL, 2-mercaptoethanol 0.05 mM, HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) 10 mM, and minimal essential medium (MEM) nonessential amino acids 0.7 × (Biochrom) at 37°C and 5% CO2 in a fully humidified atmosphere in 6-well plates at 1 × 106cells/mL.
Analysis of apoptosis
All flow cytometry analyses were made on a Coulter Epics XL cytofluorometer.
Analysis of annexin V binding to phosphatidylserine on the cell surface
Cells to be examined for annexin V expression were washed with phosphate-buffered saline (PBS) and resuspended in 500 μL binding buffer (Annexin V–FITC Kit, Immunotech, Marseille, France), containing 1 μL annexin V–fluorescein isothiocyanate (FITC) stock and 5 μL 20 μg/mL phosphatidylinositol (PI) to determinate the phosphatidylserine (PS) exposure on the outer plasma membrane. After incubation for 10 minutes at room temperature in a light-protected area, the specimens were quantified by flow cytometry, acquiring 5000 events.
Analysis of mitochondrial membrane potential
Incorporation of the cationic lipophilic dye DiOC6into the mitochondria is proportional to the mitochondrial transmembrane potential Δψ. Cells were incubated for 30 minutes with 20 nM DiOC6(3) at 37°C, then washed once in PBS, and subsequently resuspended in 500 μL binding buffer (annexin V–FITC kit, Immunotech), containing 5 μL of 20 μg/mL PI. Cells were analyzed via flow cytometry, acquiring 5000 events.
PI-3K assay
Activity of PI-3K in CLL and in Ba/F3 cells was measured as described previously,27 with minor modifications. Protein (200 μg) from the total cell lysate was immunoprecipitated with anti-p85 PI-3K antibody in the lysis buffer for 2 hours at 4°C. Immunoprecipitates were collected by adding protein A-Sepharose beads for 1 hour at 4°C with gentle agitation. Then beads were washed twice in lysis buffer, twice in buffer containing 0.1 M Tris [tris(hydroxymethyl)aminomethane]/HCl (pH 7.4) and 0.5 M LiCl, and finally in 0.15 M NaCl and 10 mM Tris/HCl (pH 7.4) and 5 mM EDTA (ethylenediaminetetraacetic acid). Kinase reaction was performed for 15 minutes at 37°C in the presence of 0.4 mg/mL PI (Sigma), 40 μM adenosine triphosphate (ATP), 30 mM MgCl2, and 10 μCi (0.37 MBq) γ-32P] ATP. The reaction was stopped by adding 20 μL 6 N HCl and the lipids were first extracted with 160 μL chloroform/methanol (1:1; vol/vol). The lipids in the organic phase were separated by thin-layer chromatography (TLC) using a silica gel 60 (Merck, Darmstadt, Germany) with chloroform/methanol/H20/NH4OH (43:38:7:5; vol/vol) and visualized by autoradiography.
Immunoblotting
To investigate cell proteins via Western blot, 1 to 10 × 107 cells were cultured in medium alone or together with LY294002 (10 μM) or Rottlerin (5 μM). After 12 and 24 hours cells were collected, washed twice in PBS, and lysed in lysis buffer (10 μM Tris/HCl, pH 7.4, 5 mM EDTA, 130 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, and 10 mg/mL each of phenantroline, aprotinin, leupeptin, and pepstatin) for 20 minutes at 4°C. Lysates were spun at 12 000 rpm for 20 minutes and supernatants were collected. Protein concentration was assessed by the Bio-Rad assay method (Bio-Rad Laboratories, Richmond, CA). Total extracts (40-100 μg/lane) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and blotting was performed on polyvinylidene difluoride (PVDF) membranes (Immobilon-P, Millipore, Schwalbach, Germany). Blots were developed using Super Signal chemoluminescent substrates from Piere Chemical (Bonn, Germany).
Akt kinase assay
Nonradioactive Akt kinase assay kit was purchased from New England Biolabs. After immunoprecipitation of Akt, the kinase reaction was performed according to the manufacturer's instructions using GSK-3 fusion protein as an exogenous substrate. The kinase reaction was analyzed by immunoblotting, using a phospho-GSK-3 antibody (Ser21/9).
PKCδ kinase assay
Cell lysates were immunoprecipitated with an anti-PKCδ antibody (BD Transduction Laboratories) and immunoprecipitates were washed 3 times with phosphorylation lysis buffer and 3 times with kinase buffer (25 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 0.5 mM EGTA, 1 mM dithiothreitol [DTT], 20 μg PS, 20 μM ATP) and were resuspended in 25 μL kinase buffer containing 5 μg histone H1 (Upstate Biotechnology) as an exogenous substrate, to which 20 μCi (0.74 MBq) γ-32P] ATP was added. The reaction was incubated for 20 minutes at room temperature and was terminated by addition of SDS sample buffer. Proteins were analyzed by SDS-PAGE and the phosphorylated form of histone H1 was detected by autoradiography.
Statistical analysis
Data from individual experiments are presented as mean ± SEM. Statistical significances were determined using the Wilcoxon signed rank test and the Mann-Whitney test as appropriate.P < .05 was considered to be statistically significant.
Results
Inhibition of PI-3K in B-CLL cells induces apoptosis in a dose-dependent manner
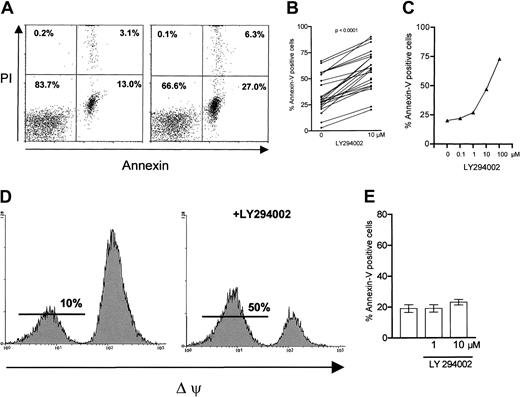
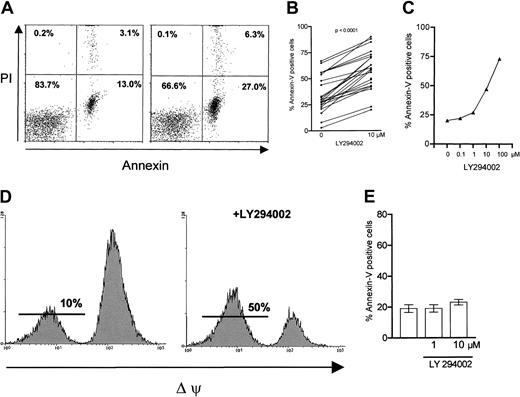
We investigated the effect of inhibition of PI-3K in B-CLL cells by its specific inhibitor LY29400228 in vitro. Cells were incubated with or without the inhibitor and apoptosis was measured by annexin/PI staining after 24 hours (Figure1A). Inhibition of PI-3K resulted in an increased number of apoptotic cells compared with spontaneous apoptosis, which ranged from 3% to 67%. An increased number of apoptotic cells could be detected in 24 of 24 tested samples (34% ± 3.5% versus 58%± 3.6%; P < .0001; Figure1B). We also considered that the concentration of cultivated CLL cells is critical for ex vivo survival of cells, depending on cell-cell interactions.29 Therefore we tested the impact of LY294002 on increasing concentrations (1-6 × 106cells/mL) of cultivated CLL cells. Apoptosis responsiveness to LY294002 was not affected by the concentration of cells ex vivo (data not shown). A dose-response analysis revealed an increase of apoptotic cells beginning at a concentration of 1 μM LY294002 with a maximum at 100 μM (Figure 1C). Because nonspecific and toxic effects cannot be ruled out at 100 μM, we chose a working concentration of 10 μM, which is known to be specific for inhibition of PI-3K. We next performed DiOC6(3) staining of the cells in the absence or in the presence of 10 μM LY294002. Inhibition of PI-3K strikingly led to a loss of the mitochondrial membrane potential (Δψ). One representative experiment is shown in Figure 1D, demonstrating an increase of DiOC6(3)-negative, apoptotic cells from 10% to 50% with LY294002 after 24 hours. We confirmed the proapoptotic effect of PI-3K inhibition by the TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuracil triphosphate [dUTP] nick-end labeling) assay (data not shown). Next we examined the effect of LY294002 on peripheral blood B cells of healthy donors. No increase in the number of apoptotic cells was detected after 24 hours of incubation with 10 μM LY294002 (Figure 1E). To exclude the possibility that the difference in apoptosis responsiveness to LY294002 between normal B cells and B-CLL cells was related to different purification processes, we purified CLL cells either by positive or by negative selection. CD19+ selection of B-CLL cells did not affect the susceptibility of cells toward apoptosis induction by LY294002 (data not shown).
Effect of PI-3K inhibition on apoptosis in B-CLL cells.
B-CLL cells (1 × 106 cells/mL) were incubated in the absence or presence of LY294002 (10 μM) for 24 hours before the indicated tests were performed. (A) Annexin/PI staining revealed an increase in apoptotic cells (right panel) compared with medium control (left panel); 1 representative experiment of 24 is shown. (B) The proapoptotic effect was demonstrated in 24 of 24 different CLL samples. (C) A representative example of dose-response data as measured by annexin/PI staining is shown. As demonstrated in panel D, apoptosis was confirmed by measuring the mitochondrial membrane potential (Δψ); PI-3K inhibition resulted in an increase of Δψ-negative, apoptotic cells. The same experiment was repeated 9 times with similar results. Peripheral B cells of healthy donors (n = 5) were resistant to the proapoptotic effect of the PI-3K inhibitor (E).
Effect of PI-3K inhibition on apoptosis in B-CLL cells.
B-CLL cells (1 × 106 cells/mL) were incubated in the absence or presence of LY294002 (10 μM) for 24 hours before the indicated tests were performed. (A) Annexin/PI staining revealed an increase in apoptotic cells (right panel) compared with medium control (left panel); 1 representative experiment of 24 is shown. (B) The proapoptotic effect was demonstrated in 24 of 24 different CLL samples. (C) A representative example of dose-response data as measured by annexin/PI staining is shown. As demonstrated in panel D, apoptosis was confirmed by measuring the mitochondrial membrane potential (Δψ); PI-3K inhibition resulted in an increase of Δψ-negative, apoptotic cells. The same experiment was repeated 9 times with similar results. Peripheral B cells of healthy donors (n = 5) were resistant to the proapoptotic effect of the PI-3K inhibitor (E).
PI-3K is constitutively activated in freshly isolated B-CLL cells
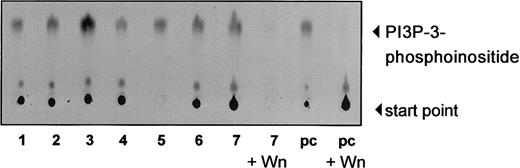
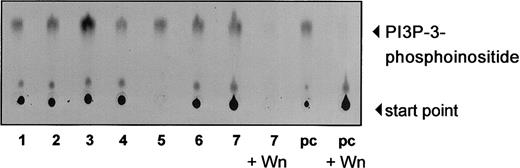
We investigated the activity of PI-3K in freshly isolated B-CLL cells. PI-3K was immunoprecipitated with anti-p85 antisera from freshly isolated B-CLL cells from 7 patients and an in vitro kinase assay was performed. Our results show that the kinase is active in all samples tested (Figure 2). To confirm the specificity of the kinase reaction, we pretreated cell lysates with wortmannin, another PI-3K inhibitor, before the kinase reaction. Wortmannin strikingly inhibited the 3-phosphoinositide (PI3P) synthesis by PI-3K. The level of activation was comparable to that of interleukin 3 (IL-3)–mediated activation of PI-3K in Ba/F3 cells, with some CLL cases showing even a stronger activity.
PI-3K is constitutively activated in B-CLL cells.
PI-3K activity was measured by incubation of anti-p85 antisera with PI and P32-γg-ATP. P32-labeled enzymatic products of PI-3K were resolved by TLC using a silica gel, as described in “Materials and methods,” and visualized by autoradiography. Total cell lysate (200 μg) of freshly isolated B-CLL cells was used for immunoprecipitation. Lanes 1 to 7 show constitutive PI-3K activity of 7 different CLL samples. IL-3–stimulated (2 ng/mL) Ba/F3 cells were used for positive control (pc). Kinase reaction could be inhibited sufficiently by adding 300 nM wortmannin (Wn) to the reaction (lanes 8 and 10).
PI-3K is constitutively activated in B-CLL cells.
PI-3K activity was measured by incubation of anti-p85 antisera with PI and P32-γg-ATP. P32-labeled enzymatic products of PI-3K were resolved by TLC using a silica gel, as described in “Materials and methods,” and visualized by autoradiography. Total cell lysate (200 μg) of freshly isolated B-CLL cells was used for immunoprecipitation. Lanes 1 to 7 show constitutive PI-3K activity of 7 different CLL samples. IL-3–stimulated (2 ng/mL) Ba/F3 cells were used for positive control (pc). Kinase reaction could be inhibited sufficiently by adding 300 nM wortmannin (Wn) to the reaction (lanes 8 and 10).
Inhibition of p38, Erk pathway, and p70S6 kinase do not affect spontaneous apoptosis in B-CLL cells
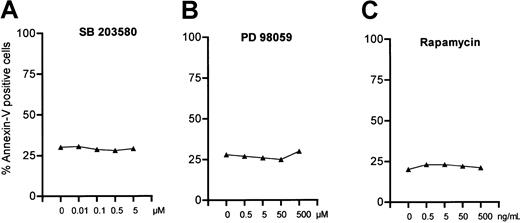
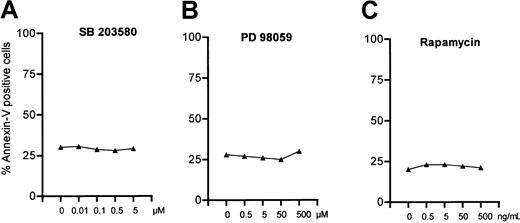
Mitogen-activated protein kinases (MAPKs) are also known to be involved in programmed cell death. Therefore, we examined whether inhibition of MAPK affects the rate of spontaneous apoptosis in B-CLL cells. Activation of JNK/SAPK and p38 MAPK is often associated with promotion of apoptosis, whereas p42/44 extracellular signaling–regulated kinase (ERK) activity inhibits apoptosis.30 Inhibition of p38 MAPK by a specific inhibitor SB20358031 did not decrease the number of apoptotic cells even at a concentration of 5 μM (Figure3A) as measured by annexin/PI staining. To answer the question if the ERK pathway is involved in apoptosis in B-CLL cells in vitro, we also incubated cells with increasing concentrations of PD98059, a specific inhibitor of the MEK/ERK pathway. PD98059 acts by blocking activation of ERK by the upstream MAPKs MEK-1 and MEK-2.32 Even at the very high concentration of 500 μM, PD98059 did not increase spontaneous apoptosis (Figure 3B). It is known that p70S6 kinase lies downstream of PI-3K and Akt.33 Therefore, we investigated whether inhibition of p70S6 kinase could mimic the proapoptotic effect of LY294002. The macrolide rapamycin blocks the activity of mammalian target of rapamycin (mTOR), which is an upstream activator of p70S6 kinase. As shown in Figure 3C, rapamycin did not affect the spontaneous apoptosis of B-CLL cells in vitro. Therefore, neither MAPKs nor p70S6 kinase seem to be involved in the defect of apoptosis in B-CLL cells.
P38-and Erk-MAPK as well as p70S6 kinase are not involved in spontaneous apoptosis of B-CLL cells.
Cells (1 × 106 cells/mL) were incubated with increasing amounts of SB203580, a specific inhibitor of p38-MAPK (A), PD98059, which inhibits Erk-MAPK pathway (B), or rapamycin, which blocks p70S6 kinase (C). Apoptosis was measured by annexin/PI staining after incubation for 24 hours. One representative result of 10 individual experiments is shown.
P38-and Erk-MAPK as well as p70S6 kinase are not involved in spontaneous apoptosis of B-CLL cells.
Cells (1 × 106 cells/mL) were incubated with increasing amounts of SB203580, a specific inhibitor of p38-MAPK (A), PD98059, which inhibits Erk-MAPK pathway (B), or rapamycin, which blocks p70S6 kinase (C). Apoptosis was measured by annexin/PI staining after incubation for 24 hours. One representative result of 10 individual experiments is shown.
LY294002-induced apoptosis can be antagonized by zvad.fmk, a pan-caspase inhibitor and by the caspase-3 inhibitor z-devd-fmk
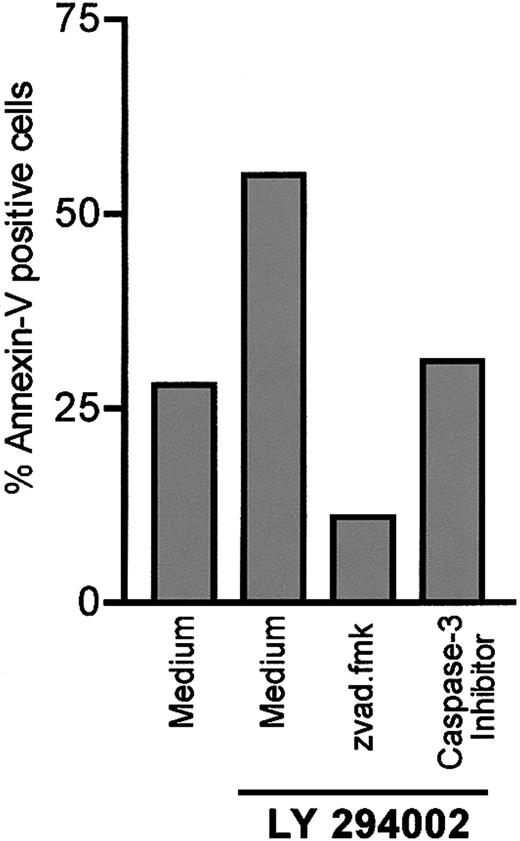
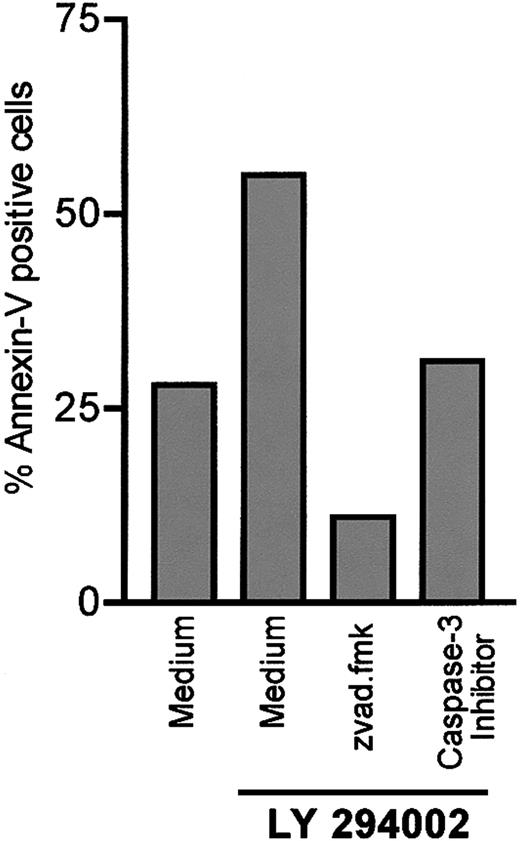
To confirm the involvement of caspases in LY294002-induced apoptosis, we investigated the impact of caspase inhibitors on B-CLL cells. It is known that zvad.fmk is a broad-spectrum caspase inhibitor targeting caspases I, III, IV, and VII.34 As shown in Figure 4, preincubation of B-CLL cells with 100 μM zvad.fmk completely antagonized LY294002-induced apoptosis. Moreover, the level of apoptotic cells was below the spontaneous rate of apoptosis even in the presence of 10 μM LY294002. A protective effect of zvad.fmk on spontaneous apoptosis was published by Bellosillo et al, who investigated the role of caspases in apoptosis of B-CLL cells.35 We next investigated the role of caspase-3 by using the caspase-3 inhibitor z-devd-fmk. Inhibition of caspase-3 also protected cells from LY294002-induced apoptosis.
Antagonism of the proapoptotic effect of LY294002 by the pan-caspase inhibitor zvad.fmk and the caspase-3 inhibitor z-devd-fmk.
B-CLL cells were incubated in medium alone or with 10 μM LY294002. Cells were preincubated either with the broad-spectrum caspase inhibitor zvad.fmk (100 μM) or the caspase-3 inhibitor z-devd-fmk (200 μM) 30 minutes prior to adding LY294002 to the culture medium. Apoptosis was detected by annexin/PI staining after 24 hours. One representative experiment of 3 is shown.
Antagonism of the proapoptotic effect of LY294002 by the pan-caspase inhibitor zvad.fmk and the caspase-3 inhibitor z-devd-fmk.
B-CLL cells were incubated in medium alone or with 10 μM LY294002. Cells were preincubated either with the broad-spectrum caspase inhibitor zvad.fmk (100 μM) or the caspase-3 inhibitor z-devd-fmk (200 μM) 30 minutes prior to adding LY294002 to the culture medium. Apoptosis was detected by annexin/PI staining after 24 hours. One representative experiment of 3 is shown.
Inhibition of PI-3K results in down-regulation of antiapoptotic proteins
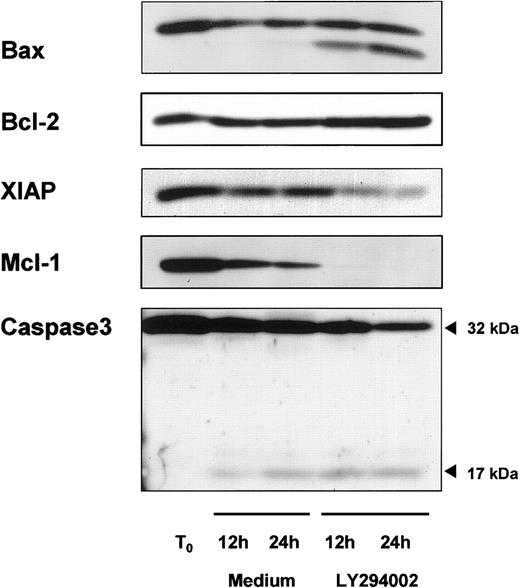
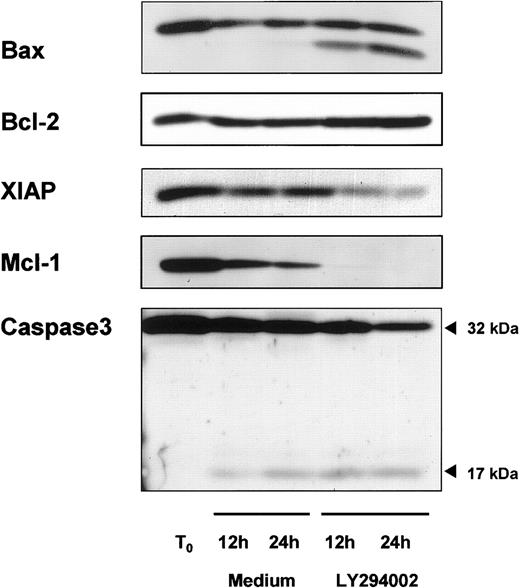
High levels of the antiapoptotic proteins bcl-2 and Mcl-1 are commonly found in circulating B-CLL cells and might play an important role in the pathophysiology of the disease. Therefore, we studied the expression of bcl-2, and its counterpart bax, and Mcl-1 during in vitro culture with or without LY294002. As shown in Figure5, freshly isolated B-CLL cells expressed high levels of bcl-2, but we did not detect any change in the level of this protein during the time course of 24 hours with or without LY294002. In contrast to bcl-2, incubation of cells with LY294002 induced a proteolytic degradation of its counterpart bax. The proapoptotic bax cleavage product became detectable within 12 hours. Mcl-1 is also a member of the bcl-2 family and high levels have been correlated with failure to achieve complete remission to chemotherapy in CLL. Inhibition of PI-3K resulted in a complete loss of the Mcl-1 protein compared with resting B-CLL cells, but a spontaneous decrease also was observed when cells were incubated in medium alone. Finally, XIAP is a member of the inhibitor of apoptosis (IAP) family, and because of its ability to directly inhibit some members of the caspase family36 it may be important in CLL. Freshly isolated B-CLL cells expressed high levels of XIAP and no spontaneous decrease in cells cultured for 24 hours in medium was observed. In contrast, incubating cells with LY294002 resulted in a decreased XIAP expression. Because caspases are known to mediate important steps in the apoptotic pathway, we next investigated procaspase-3 activity. A procaspase-3 cleavage fragment was detected in resting B-CLL cells as well as in cells incubated with LY294002, but inhibition of PI-3K resulted in a stronger cleavage of procaspase-3 (Figure 5).
Modulation of proapoptotic and antiapoptotic proteins in response to PI-3K inhibition.
Representative immunoblot data show regulation of proapoptotic and antiapoptotic proteins. B-CLL cells were cultured with or without 10 μM LY294002. Cells were harvested after 12 and 24 hours and whole cell lysates were prepared. A protein content of 40 μg was subjected to SDS-PAGE/immunoblot analysis by the use of specific antibodies for Bax, Bcl-2, XIAP, Mcl-1, and caspase-3.
Modulation of proapoptotic and antiapoptotic proteins in response to PI-3K inhibition.
Representative immunoblot data show regulation of proapoptotic and antiapoptotic proteins. B-CLL cells were cultured with or without 10 μM LY294002. Cells were harvested after 12 and 24 hours and whole cell lysates were prepared. A protein content of 40 μg was subjected to SDS-PAGE/immunoblot analysis by the use of specific antibodies for Bax, Bcl-2, XIAP, Mcl-1, and caspase-3.
PKB/Akt is not constitutively activated in B-CLL cells
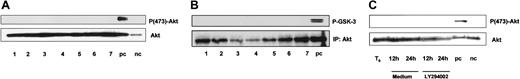
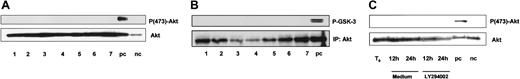
Protein kinase B/Akt is activated by PI-3K via the 3-phosphoinosititde-dependent kinase PDK1. Because of its important role in mediating cell survival, we investigated whether Akt is constitutively activated in freshly isolated B-CLL cells. Maximal activation of Akt requires phosphorylation of the residues Thr308 and Ser473.37 As shown in Figure6A, Akt is expressed in all samples tested but no phosphorylation of Akt at Ser473 was detected. Furthermore, we did not detect any phosphorylation of Thr308 (data not shown). Next we performed an Akt in vitro kinase assay to confirm that Akt is not active in B-CLL cells. Again we failed to detect any activity of Akt (Figure 6B). To rule out the possibility of an in vitro activation of Akt, we incubated cells for 24 hours with or without LY294002 and repeated Western blot. Akt expression remained stable during the time course of 24 hours but no phosphorylation occurred in vitro (Figure 6C).
PKB/Akt is expressed but not constitutively phosphorylated in B-CLL.
B-CLL cells were lysed immediately after separation from peripheral blood. (A) Whole protein lysates (100 μg) from 7 different patients were analyzed by immunoblotting with the antibodies as indicated. NIH-3T3 cells treated with platelet-derived growth factor (PDGF) served as a positive control (pc); without PDGF as a negative control (nc). (B) Akt in vitro kinase assay was performed after immunoprecipitation of Akt from 7 different patients; GSK-3 fusion protein served as exogenous substrate for Akt. Kinase reaction was analyzed by immunoblotting with mAb specific for phospho-GSK-3 (Ser21/9). (C) To rule out in vitro phosphorylation of Akt, B-CLL cells were incubated with or without 10 μM LY294002 and lysed after 12 and 24 hours.
PKB/Akt is expressed but not constitutively phosphorylated in B-CLL.
B-CLL cells were lysed immediately after separation from peripheral blood. (A) Whole protein lysates (100 μg) from 7 different patients were analyzed by immunoblotting with the antibodies as indicated. NIH-3T3 cells treated with platelet-derived growth factor (PDGF) served as a positive control (pc); without PDGF as a negative control (nc). (B) Akt in vitro kinase assay was performed after immunoprecipitation of Akt from 7 different patients; GSK-3 fusion protein served as exogenous substrate for Akt. Kinase reaction was analyzed by immunoblotting with mAb specific for phospho-GSK-3 (Ser21/9). (C) To rule out in vitro phosphorylation of Akt, B-CLL cells were incubated with or without 10 μM LY294002 and lysed after 12 and 24 hours.
PKCδ is constitutively activated in freshly isolated B-CLL cells: dependence on PI-3K activity
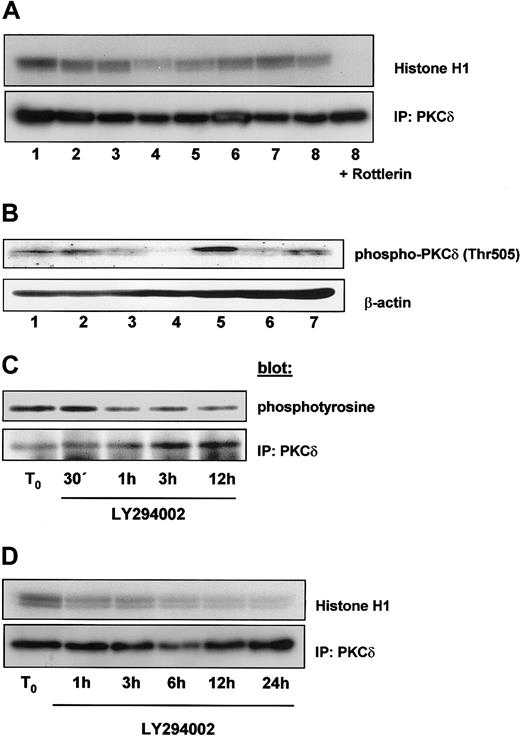
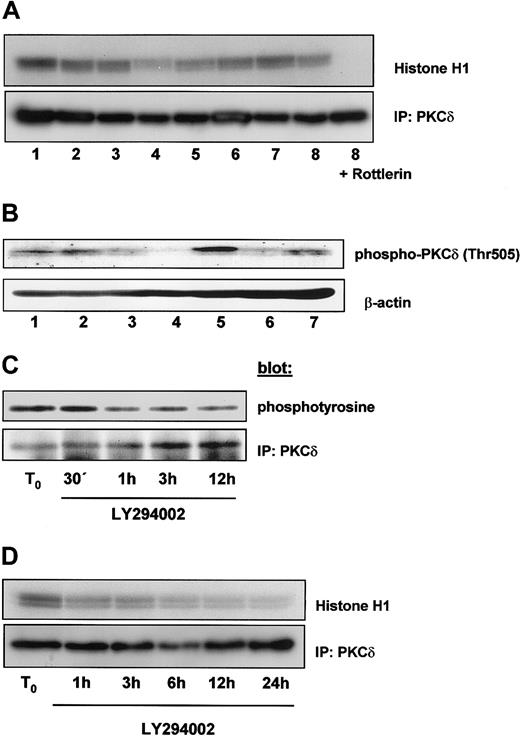
PI-3K signaling is also linked to novel and atypical PKC isoforms. One novel isoform is PKCδ, which is involved in the regulation of cell cycle and apoptosis. PKCδ is associated with PI-3K following cytokine stimulation and is activated by PDK1 at Thr505. Therefore, we examined whether PKCδ is constitutively activated in B-CLL cells. Cell lysates of freshly isolated B-CLL cells were immunoprecipitated with an antibody against PKCδ, and in vitro kinase assay was carried out on immunoprecipitates using histone H1 as an exogenous substrate. We detected a constitutive activation of PKCδ on 8 different samples; kinase activity was inhibited by the addition of Rottlerin (5 μM) to the kinase reaction (Figure 7A, lane 9). Next we examined whether PKCδ was also constitutively phosphorylated on Thr505. Figure 7B shows constitutive phosphorylation of PKCδ at Thr505. The level of phosphorylation was different in individual samples. To investigate the dependence of tyrosine phosphorylation of PKCδ on PI-3K activity, we incubated cells with LY294002 over a time period of 12 hours and measured tyrosine phosphorylation after PKCδ immunoprecipitation. As shown in Figure 7C, inhibition of PI-3K led to a reduction of PKCδ tyrosine phosphorylation, becoming detectable after 1 hour. Accordingly, PKCδ activity declined after PI-3K inhibition by LY294002 as shown in Figure7D. These data demonstrate that Rottlerin inhibits PKCδ activity and that inhibition of PI-3K negatively affects tyrosine phosphorylation and activity of PKCδ in B-CLL cells.
PKCδ is constitutively activated in B-CLL cells.
(A) A sample of 300 μg total protein content of 8 different freshly isolated B-CLL cells was immunoprecipitated with an antibody against PKCδ and in vitro kinase assay was performed in the absence (lanes 1-8) or presence (lane 9) of Rottlerin using histone H1 as an exogenous substrate. Proteins were analyzed by SDS-PAGE and a phosphorylated form of histone H1 was detected by autoradiography. (B) A sample of 100 μg total protein content of 7 different B-CLL cells was analyzed by immunoblot with an antiphosphothreonine 505 antibody specific for PKCδ. Samples from panel A were different from those shown in panel B. To investigate the influence of PI-3K activity on PKCδ, cells were incubated with 10 μM LY294002 and lysed after the indicated time period. Then PKCδ was immunoprecipitated with a specific antibody. (C) Tyrosine phosphorylation was detected by using an antiphosphotyrosine 4G10 antibody. (D) PKCδ kinase activity was measured by a in vitro kinase assay as described in (A).
PKCδ is constitutively activated in B-CLL cells.
(A) A sample of 300 μg total protein content of 8 different freshly isolated B-CLL cells was immunoprecipitated with an antibody against PKCδ and in vitro kinase assay was performed in the absence (lanes 1-8) or presence (lane 9) of Rottlerin using histone H1 as an exogenous substrate. Proteins were analyzed by SDS-PAGE and a phosphorylated form of histone H1 was detected by autoradiography. (B) A sample of 100 μg total protein content of 7 different B-CLL cells was analyzed by immunoblot with an antiphosphothreonine 505 antibody specific for PKCδ. Samples from panel A were different from those shown in panel B. To investigate the influence of PI-3K activity on PKCδ, cells were incubated with 10 μM LY294002 and lysed after the indicated time period. Then PKCδ was immunoprecipitated with a specific antibody. (C) Tyrosine phosphorylation was detected by using an antiphosphotyrosine 4G10 antibody. (D) PKCδ kinase activity was measured by a in vitro kinase assay as described in (A).
Inhibition of PKCδ by Rottlerin induces apoptosis in B-CLL cells and not in peripheral B cells of healthy controls
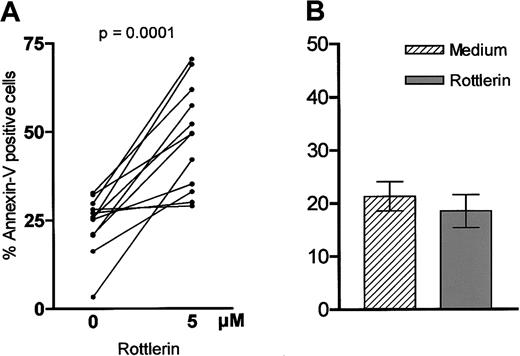
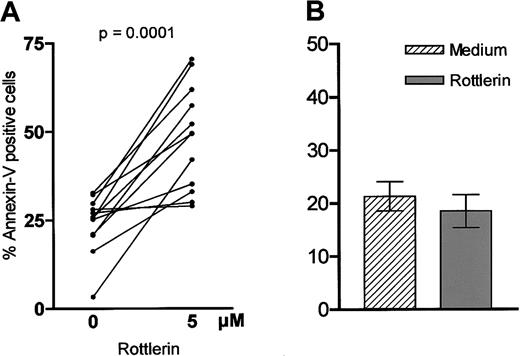
We next analyzed the influence of inhibition of PKCδ on apoptosis in B-CLL cells. Cells were incubated with or without the PKCδ inhibitor Rottlerin38 for 24 hours prior to measuring apoptosis. Interestingly, Rottlerin induced apoptosis in B-CLL cells (Figure 8A) and in most instances the effect was even stronger compared with PI-3K inhibition (data not shown). To support the importance of PKCδ and the specificity of its inhibitor Rottlerin, we repeated the experiments with the PKC inhibitor Gö 6976, which is known to specifically inhibit the Ca++-dependent PKC isoforms α and -β39; Gö 6976 did not show any impact on the percentage of apoptotic B-CLL cells in vitro (data not shown). Next we investigated the impact of PKCδ inhibition on peripheral B cells of healthy donors. In contrast to B-CLL cells, Rottlerin did not induce apoptosis in normal B cells. Furthermore, we saw a trend toward an antiapoptotic effect on nonmalignant cells, but no statistical significance was reached (P = .125; Figure 8B).
Influence of PKCδ inhibition on viability of B-CLL cells.
Cells (1 × 106 cells/mL) were incubated with or without Rottlerin (5 μM), which specifically inhibits PKCδ. After 24 hours apoptosis was measured by annexin/PI staining. Panel A shows the results of 12 different experiments. The proapototic effect was compared with peripheral B cells of 5 healthy donors (B).
Influence of PKCδ inhibition on viability of B-CLL cells.
Cells (1 × 106 cells/mL) were incubated with or without Rottlerin (5 μM), which specifically inhibits PKCδ. After 24 hours apoptosis was measured by annexin/PI staining. Panel A shows the results of 12 different experiments. The proapototic effect was compared with peripheral B cells of 5 healthy donors (B).
Discussion
Constitutively activated signaling pathways are a common finding in hematologic malignancies.40-42 They might also play an important role in the disturbance of apoptosis in B-CLL. Important features are the high level of NF-κB activity in unstimulated CLL cells compared with nonmalignant human B cells8 as well as constitutive phosphorylation of STAT1 on Ser727.
In the present work we have shown for the first time that PI-3K is constitutively activated in CLL cells and that specific inhibition of this kinase increases apoptosis in B-CLL cells. PI-3K is a ubiquitously expressed protein kinase that is involved in the regulation of normal and neoplastic cell growth. Many factors that are important for the development and survival of normal B cells use the PI-3K pathway, including CD40 and BCR ligation.11 Mice that lack the p85 subunit of PI-3K exhibit profound defects of B-cell development, as well as a diminished proliferation response and survival.43 Some of the cytokines that protect B-CLL cells from apoptosis, such as IL-4, transduce their effects through PI-3K in normal B cells.44 Recently, it has been published that survival effects of IL-4 and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) on B-CLL cells are mediated via the PI-3K pathway.45 Because of its importance in many critical signaling pathways, it is not surprising that the PI-3K pathway is involved in the development of solid tumors46 and hematologic malignancies such as multiple myeloma.47 Several different possibilities have to be considered to explain the constitutive activation of PI-3K in B-CLL cells: (1) PI-3K activity is related to an intrinsic defect of B-CLL cells leading to a permanent activation of downstream antiapoptotic factors; (2) PI-3K is activated by an extrinsic, humoral factor mediating survival signals to the cell; and (3) PI-3K regulation is disturbed in CLL cells resulting in a long-lasting activation after receptor ligation. The resistance of normal B cells toward apoptosis induced by PI-3K inhibition demonstrates that kinase activation is critical for B-CLL cells but not for normal resting peripheral B cells. This difference is supported by the results of Aagaard-Tillery and Jelinek11 in human B cells, because they failed to detect any kinase activity in unstimulated peripheral B cells; PI-3K activity became detectable only after cross-linking of membrane-bound immunoglobulin or CD40 ligation.
Despite the crucial role of PI-3K with respect to proliferation and survival of B cells, little is known about the events following PI-3K activity. The serine/threonine kinase Akt, or PKB, is the best characterized downstream kinase of PI-3K. The importance of Akt in CLL cells with respect to protection of apoptosis has recently been investigated. Autologous plasma has been shown to protect B-CLL cells from spontaneous or cytotoxic-induced apoptosis; this relies on a PI-3K/Akt–dependent pathway.48 In contrast to other reports, Bernal et al have demonstrated that engagement of surface IgM on B-CLL cells also improves cell survival; this occurs via activation of PI-3K and phosphorylation of Akt.49 In this recently published work no toxic effect or change in viability was observed when CLL cells were preincubated with LY294002 alone, even at a higher concentration of 75 μM. This appears to be in conflict with our present work; one explanation for this discrepancy can be the time of preincubation with the PI-3K inhibitor prior to measuring apoptosis. In that particular experiment the time of incubation was not described. In our experiments we failed to detect any proapoptotic effect of LY294002 before 8 hours of incubation (data not shown). Another explanation might be the method used to measure apoptosis; PI staining might be too insensitive to reveal slight differences in the number of apoptotic cells. Again, as mentioned above, we confirmed the proapoptotic effect of PI-3K inhibition by several different methods. Although PI-3K/Akt is involved in survival signals in CLL cells mediated by factors that are known to use the PI-3K/Akt pathway, our results have shown that Akt is not constitutively activated due to the constitutive PI-3K activity.
In the past, observations have been made showing that PI 3,4-P2 and PI 3,4,5-P3 can also activate novel and atypical PKC isoforms in vitro.19 This occurs in context with a marked increase in the autophosphorylation of PKCδ, ε, and η. With respect to PKCδ, it has been demonstrated that the PKB kinase PDK1 is responsible for phosphorylation of Thr505 in the activation loop of the enzyme.50 It has to be considered that this phosphorylation, unlike that of corresponding threonines in other PKC isoforms, is not essential for the formation of functional PKCδ as shown by site-directed mutagenesis,51 but PKCδ activity is increased approximately 2-fold by PDK1 phosphorylation. However, phosphorylation of Thr505 may be necessary for other purposes, such as protein-protein interactions. The significance of tyrosine phosphorylation of PKCδ also needs to be further determined. According to in vitro studies, it seems to depend on the substrate whether PKC activity is elevated or reduced by tyrosine phosphorylation.52 In the present work, we have shown that inhibition of PI-3K also reduces the level of phosphotyrosine of PKCδ in CLL cells, according to a decrease of enzyme activity. Despite the uncertainty about the tyrosine and threonine phosphorylation of PKCδ, we have shown a biologic relevance of PKCδ exclusively in CLL, whereas inhibition of the kinase does not affect apoptosis in peripheral B cells of healthy donors. One of the most important tasks in understanding the role of PKCδ in the pathogenesis of CLL is the search for physiologic substrates and associated signaling pathways. PKCδ is linked to serine phosphorylation of STAT proteins in several different cell types53 as well as to NF-κB activation in human neutrophils.54 Because both pathways are constitutively activated in B-CLL cells, further in vitro studies are needed and experiments are in progress in our laboratory.
An increasing number of publications have demonstrated the importance of PKC in CLL. PKC activation in CLL cells has been implicated with the suppression of either spontaneous or drug-induced apoptosis. Activation of PKC by phorbol ester like TPA is able to prevent dexamethasone- or chemotherapy-induced apoptosis.35,55 Bryostatin-1 is a member of the macrocyclic lactones that structurally mimic the PKC-activating second messenger diacylglycerol. Incubation of CLL cells with bryostatin-1 up-regulates the protein levels of Mcl-1 and XIAP in accordance with an enhanced apoptosis resistance.56 On the other hand, it has been shown that nonselective inhibition of PKC by UCN-01 induces apoptosis in B-CLL cells.57 However, it has not been shown which isoform of PKC is important in the regulation of apoptosis. We conclude from our data that PKCδ might have a predominant role in PKC-mediated prevention of apoptosis, because we failed to detect any impact on apoptosis when B-CLL cells were incubated with inhibitors known to be specific for inhibition of other isoforms (data not shown).
The knowledge of disturbed signaling pathways can open new opportunities in the treatment of a disease, as has been impressively shown for chronic myeloid leukemia.58 Despite the lack of a unique pathognomonic feature in CLL, different cellular alterations could end up in the same signaling pathway, causing cell cycle arrest or prevention of apoptosis. Here we have shown that PI-3K as well as PKCδ are involved in apoptosis in CLL. To our knowledge, inhibition of PI-3K in the treatment of a malignant disease has never been investigated in a clinical trial. Because PI-3K is ubiquitously expressed and involved in so many physiologic events, this does not seem to be a feasible approach. In contrast, PKC inhibitors already have been tested in clinical trials59 and have shown antiproliferative activity against various tumors in vitro.60 Bryostatin, which acts as a PKC inhibitor when cells are exposed for a long period, has shown antileukemic activity in a phase II trial in patients with low-grade non-Hodgkin lymphoma and CLL.61 Targeting signaling pathways seems to be a breakthrough in the treatment of an incurable disease. Inhibition of PKCδ in particular may be a promising novel approach for the treatment of CLL.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-02-0539.
Supported by a research grant from the Technical University of Munich (KKF 15-00) and a grant from the Deutsche Forschungsgemeinschaft DE 771.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ingo Ringshausen, Third Department of Medicine, Technical University of Munich, Ismaninger Str 15, 81675 Munich, Germany; e-mail: i.ringshausen@lrz.tum.de.