The anemia of chronic disease is a prevalent, poorly understood condition that afflicts patients with a wide variety of diseases, including infections, malignancies, and rheumatologic disorders. It is characterized by a blunted erythropoietin response by erythroid precursors, decreased red blood cell survival, and a defect in iron absorption and macrophage iron retention, which interrupts iron delivery to erythroid precursor cells. We noted that patients with large hepatic adenomas had severe iron refractory anemia similar to that observed in anemia of chronic disease. This anemia resolved spontaneously after adenoma resection or liver transplantation. We investigated the role of the adenomas in the pathogenesis of the anemia and found that they produce inappropriately high levels of hepcidin mRNA. Hepcidin is a peptide hormone that has been implicated in controlling the release of iron from cells. We conclude that hepcidin plays a major, causative role in the anemia observed in our subgroup of patients with hepatic adenomas, and we speculate that it is important in the pathogenesis of the anemia of chronic disease in general.
Introduction
The erythroid bone marrow requires a large, continuous supply of iron to make normal red blood cells. This iron comes from 2 sources. A small portion enters the body each day through intestinal absorption of dietary iron. A much larger fraction becomes available through recycling of iron from senescent red blood cells. This iron recycling is carried out by specialized reticuloendothelial macrophages that engulf aged erythrocytes, lyse them, and catabolize their hemoglobin. The macrophages store part of the recovered iron and export the rest to the plasma, where it is taken up by transferrin.
The anemia of chronic disease is an acquired disorder seen in patients with a variety of inflammatory disorders. The pathogenesis of this anemia has been attributed to deficiencies at multiple steps in erythropoiesis, including a blunted response to erythropoietin, decreased survival of mature red blood cells, and decreased iron availability.1 The low serum iron and accumulation of iron in the reticuloendothelial cells of these patients is thought to result from the retention of iron by reticuloendothelial macrophages and decreased intestinal iron absorption.2,3 This impairment of iron delivery by macrophages and enterocytes is thought to be part of a host defense mechanism to fight infection and cancer.4,5 However, it also leads to a deficiency of iron available for erythropoiesis.6 Early in their course, patients with the anemia of chronic disease have normal body iron stores and a mild, normocytic anemia that results from impaired iron recycling. Too much iron is retained in reticuloendothelial macrophages and, consequently, serum iron and serum transferrin saturation values are low. Over time the impaired intestinal iron absorption associated with the anemia of chronic disease leads to frank iron deficiency, and the anemia becomes microcytic.
The molecular basis of the anemia of chronic disease has not been elucidated. Cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interferons are hypothesized to be involved in the maintenance of red blood cell production or stability.1 Many studies have shown a correlation between inflammation, elevated circulating cytokines, and anemia in patients and in mice, but whether these inflammatory cytokines act alone or regulate other pathways that are important for red blood cell production is unclear. In vitro studies have shown that treatment of cultured cells with cytokines can alter ferritin and transferrin receptor expression and iron-responsive protein activity in macrophages,7 but direct evidence for a role of those molecules in regulating iron egress is lacking.2 8
Recently, it was speculated that a small peptide hormone might be involved in the pathogenesis of this disorder.9 Hepcidin is a 20–, 22–, or 25–amino acid peptide that is cleaved from a larger precursor. It is produced in the liver and detectable in serum and urine.10,11 Hepcidin has intrinsic antimicrobial activity, and its expression increases in response to inflammatory stimuli.12,13 Animals that inadvertently lost expression of hepcidin as a result of targeted disruption of USF2, a neighboring gene, developed hepatic iron overload associated with decreased iron in tissue macrophages,14 whereas animals that retained hepcidin despite loss of USF2 did not.13 Hepcidin mRNA expression is markedly increased in some mouse models of iron overload, raising the possibility that it might be part of a compensatory response to limit iron absorption.12 This conclusion is strongly supported by the recent finding that transgenic mice that constitutively express hepcidin develop severe iron deficiency anemia.13Together, these observations make hepcidin a very attractive candidate for a direct mediator in the pathogenesis of the anemia of chronic disease by acting as a negative regulator of intestinal iron absorption and macrophage iron release.9 However, there was previously little experimental data to support this notion.
We have found evidence of a role for hepcidin in a remarkable iron refractory anemia observed in a cohort of type 1a glycogen storage disease (GSD1a) patients. GSD1a is caused by deficiency of glucose-6-phosphatase, which catalyzes the terminal reactions of both glycogenolysis and gluconeogenesis, converting glucose-6-phosphate to glucose. Lack of this enzyme causes an inability to maintain normal glucose homeostasis; severe hypoglycemia occurs if glucose is not provided continuously.15 Continuous provision of a glucose source ameliorates the biochemical abnormalities, allowing patients to survive into adulthood. Prolonged survival has led to the emergence of serious long-term complications, including chronic hepatic inflammation, hepatic adenomas, focal segmental glomerulosclerosis, nephrocalcinosis, and anemia.16 17
Although many of our older patients with GSD1a have mild anemia, 6 patients (16%) have developed severe, unremitting anemia. These individuals did not respond to oral iron supplementation and showed a delayed, partial response to replacement doses of intravenous iron dextran,17 as seen in the anemia of chronic disease. Their anemia is unrelated to metabolic control of GSD1a but is associated with the presence of large hepatic adenomas. In fact, those patients with severe anemia had the greatest tumor burdens of all of our patients with GSD1a.
We observed spontaneous resolution of anemia in an 18-year-old patient who underwent resection of her large, isolated hepatic adenoma. Studies of her liver showed inappropriately high expression of hepcidin mRNA in adenoma tissue. Most adenomas in GSD1a patients are not resectable. However, we observed similar, inappropriate hepcidin mRNA expression in adenoma tissue from another GSD1a patient who experienced resolution of his anemia following an allogeneic liver transplantation. We propose that aberrant high level hepcidin mRNA expression played a direct role in causing the anemia in these patients.
Materials and methods
Tissue preparation and analysis
Tissue samples from the adenoma and the unaffected liver were frozen in liquid nitrogen immediately after resection. Samples were also fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. We isolated total RNA using Ultraspec (Biotecx Laboratories, Houston, TX) per the manufacturer's instructions. We obtained formalin-fixed and paraffin-embedded tissue from another patient with GSD1a who underwent a liver transplantation for complications of multiple, large hepatic adenomas. The investigative review board of Children's Hospital Boston approved the use of all tissue samples.
Human cytokine expression array
We prepared α33P deoxycytidine triphosphate (dCTP)–labeled (Amersham Pharmacia Biotech, Piscataway, NJ) cDNA using 5 μg total RNA from adenoma and unaffected liver tissue using human cytokine-specific primers (R&D Systems, Minneapolis, MN). We used the labeled cDNA samples to probe duplicate nylon human cytokine expression arrays (R&D Systems) containing cDNA molecules representing 180 different cytokine genes according to the manufacturer's instructions. We quantified gene expression by phosphorimager analysis and normalized to cyclophilin or L19.
Northern blot analysis
We purchased control liver RNA from a 27-year-old male subject (Clontech, Palo Alto, CA) and prepared Northern blots according to standard procedures using 5 μg total RNA per lane. We probed blots with α32P- dCTP–labeled polymerase chain reaction (PCR) products representing human hepcidin, mouse hepcidin, human β-actin, or mouse β-actin. We quantified the relative expression of hepcidin in each RNA sample by phosphorimager analysis and normalized to β-actin. Nonheme iron was quantified using a colorimetric assay as described previously.18
In situ hybridization
We prepared digoxigenin-labeled probes for in situ hybridization as described previously.19 We performed in situ hybridization essentially as described,19 with minimal modifications. We prehybridized samples at 56°C for 3 hours and then added 1.5 ng riboprobe to hybridize at 55°C overnight. The highest stringency of posthybridization washes was 60°C in 0.1 × sodium citrate sodium chloride (SSC) (1 × SSC is 15 mM trisodium citrate, 150 mM NaCl) with β-mercaptoethanol and ethylenediaminetetraacetic acid (EDTA) for 2 hours. We applied antibody to digoxigenin for 2 hours at room temperature.
Results
GSD1a patients with large hepatic adenomas have severe iron refractory anemia
Severe anemia in older GSD1a patients is characterized by microcytosis, increased red cell distribution width, low serum iron concentrations, and very low transferrin saturations (Table1). Oral iron administration did not correct this anemia in any of the patients, and intravenous iron dextran administration allowed for only a partial correction of the anemia over an extended time. Taken together, our findings indicate that the GSD1a patients with large adenomas have a severe defect in reticuloendothelial iron recycling accompanied by systemic iron deficiency.
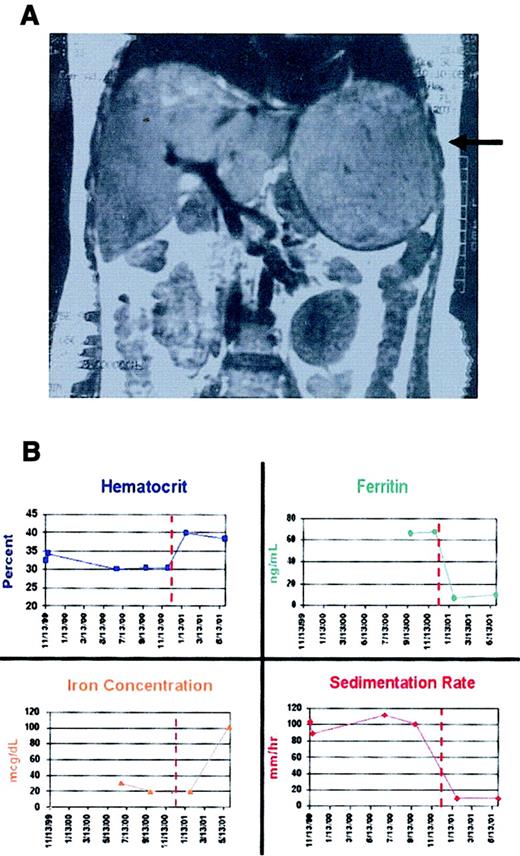
Patient A presented at 8 months of age (February 1982) with a hypoglycemic coma. A diagnosis of GSD1a was made by liver biopsy and subsequently confirmed by mutation analysis. She was managed on a regimen of frequent feeds during the day and nocturnal nasogastric feeds until June 1998, when uncooked cornstarch replaced overnight nasogastric feeds. At that time, she had a normal hematocrit level and normal iron studies. An abdominal ultrasound showed no evidence of focal hepatic lesions. A year later, at the time of a routine follow-up evaluation, she had a microcytic anemia, and a single, large hepatic adenoma was discovered by ultrasound examination. The anemia persisted, and follow-up magnetic resonance imaging (MRI) obtained 3 months later demonstrated further enlargement of the hepatic adenoma (Figure 1A).
Laboratory findings in patient A before and after adenoma resection.
(A) An MRI scan performed prior to resection (August 30, 2000) shows a 13 × 14 cm adenoma in the left lobe of A's liver (arrow). (B) Individual panels illustrate changes in hematocrit, serum ferritin concentration, serum iron concentration, and erythrocyte sedimentation rate for approximately 1 year before and 6 months after resection (performed December 15, 2000). The serum ferritin was initially above the normal range but fell to subnormal levels after the tumor was resected, and erythroid iron utilization returned to normal.
Laboratory findings in patient A before and after adenoma resection.
(A) An MRI scan performed prior to resection (August 30, 2000) shows a 13 × 14 cm adenoma in the left lobe of A's liver (arrow). (B) Individual panels illustrate changes in hematocrit, serum ferritin concentration, serum iron concentration, and erythrocyte sedimentation rate for approximately 1 year before and 6 months after resection (performed December 15, 2000). The serum ferritin was initially above the normal range but fell to subnormal levels after the tumor was resected, and erythroid iron utilization returned to normal.
The adenoma was resected in December 2000. The anemia resolved spontaneously within 6 weeks even though no blood transfusions, bone marrow modulators, or iron had been administered (Figure 1B). Over the ensuing months, patient A's iron studies normalized and have remained normal for 1 year.
GSD1a adenoma tissue does not sequester iron
Removal of patient A's large adenoma promptly corrected her anemia, which suggested that the adenoma had been hindering iron utilization. To determine whether the adenoma was storing iron at the expense of the bone marrow, we used Perl Prussian blue stain to compare the nonheme iron load of the adenoma versus the unaffected liver. Neither unaffected liver nor adenoma tissue contained significant stainable iron, indicating that liver iron stores were depleted and that the adenoma was not sequestering the metal.
GSD1a adenoma tissue and unaffected liver tissue have comparable cytokine expression profiles
We considered the possibility that the adenoma secreted a soluble factor that inhibited iron absorption and macrophage iron release. Cytokines have been postulated to play a direct role in the pathogenesis of the anemia of chronic disease.8 To evaluate cytokine expression by the adenoma, we probed a 180-gene cytokine cDNA array with labeled RNA samples from unaffected liver or adenoma tissue. We looked for evidence of genes that were differentially expressed between the 2 tissue types and for differential expression of genes that have been suggested to be involved in the anemia of chronic disease (Table2). We found that no cytokine gene showed more than a 2-fold increase in expression in the adenoma tissue when compared with unaffected liver tissue. There was a slight increase in expression of several genes consistent with a host inflammatory response against the adenoma. IL-13 and IL-16 were slightly increased in the adenoma. Importantly, there was no induction of TNF, IL-1, IL-6, or interferons in the adenoma tissue. We confirmed the relative expression of some genes in the array by Northern blot analysis (data not shown).
Hepcidin mRNA expression is turned off in mice with iron deficiency anemia
We next considered the possibility that hepcidin might be aberrantly regulated in the GSD1a adenomas. Hepcidin is a reasonable candidate because it is a plasma factor produced by hepatocytes that is hypothesized to decrease cellular iron egress. To determine how hepcidin mRNA expression responds to changes in iron status and anemia, we examined liver samples from wild-type mice and mice with genetic defects that interrupt iron transport and cause anemia. We and others have previously established that the mouse is an excellent animal model for understanding human iron metabolism.20 It had already been shown that hepcidin mRNA levels increase in hepatocytes of mice with iron overload,12 that loss of hepcidin mRNA expression results in iron overload,14 and that inappropriately increased hepcidin expression results in severe iron deficiency in mice.13
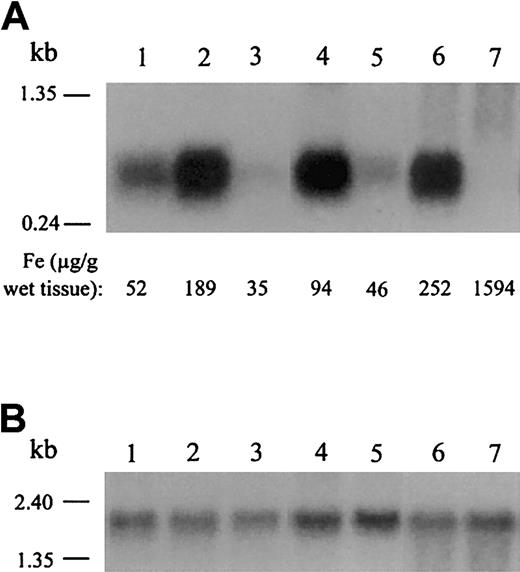
We found that hepcidin mRNA expression is markedly decreased in the livers of iron-deficient, anemic mutant mice when compared with wild-type mice of the same genetic background (Figure2). Mice with the sla mutation have a deletion in the gene encoding hephaestin,21resulting in iron deficiency anemia due to decreased intestinal iron absorption. Mice homozygous for the mk mutation have severe loss of function of the iron transporter divalent metal ion transporter 1 (DMT1; formerly Nramp2, DCT1),22 resulting in anemia due to decreased intestinal iron absorption and decreased iron uptake by erythroid precursors. Mice with the hpx mutation have aberrant splicing of the transferrin gene,23 resulting in decreased iron uptake by erythroid precursors despite systemic iron overload. We also found that hepcidin mRNA expression is decreased in the livers of anemic hpx/hpx mice when compared with nonanemic littermates (Figure 2). This is important because decreased hepcidin mRNA expression occurred despite significant iron loading of the hepatocytes, indicating that decreased hepcidin mRNA expression is associated with iron-restricted erythropoiesis, even in the setting of abundant iron stores.
Northern analysis of hepcidin mRNA expression in mouse models of anemia.
Northern analysis of hepcidin (A) and β-actin (B) expression in mouse liver tissue. Quantitative measurements of nonheme liver iron are recorded for each mouse at the bottom of panel A. We determined β-actin expression to normalize for RNA loading. Hepcidin mRNA expression in livers of C57BL/6J mice (lane 1) was less than that of 129/SvEvTac mice (lane 2). This result is consistent with the observation that wild-type 129/SvEvTac mice generally have higher iron stores than wild-type C57BL/6J mice. Anemic sla/Y mice (lane 3) have low liver iron stores and low hepcidin levels when compared with wild-type mice of the same background strain (C57BL/6J; lane 1). Nonanemic mk/+ mice (lane 4) have more liver iron and express more hepcidin than anemic mk/mk homozygotes (lane 5), which have low iron stores and microcytic anemia. Homozygoushpx/hpx mice cannot efficiently deliver iron to developing red blood cells, resulting in severe anemia despite tissue iron overload (1594 μg iron per gram of liver). These mice have decreased hepcidin mRNA expression (lane 7) when compared with nonanemic littermates (lane 6). These results are representative of 2 experiments without significant variation between experiments.
Northern analysis of hepcidin mRNA expression in mouse models of anemia.
Northern analysis of hepcidin (A) and β-actin (B) expression in mouse liver tissue. Quantitative measurements of nonheme liver iron are recorded for each mouse at the bottom of panel A. We determined β-actin expression to normalize for RNA loading. Hepcidin mRNA expression in livers of C57BL/6J mice (lane 1) was less than that of 129/SvEvTac mice (lane 2). This result is consistent with the observation that wild-type 129/SvEvTac mice generally have higher iron stores than wild-type C57BL/6J mice. Anemic sla/Y mice (lane 3) have low liver iron stores and low hepcidin levels when compared with wild-type mice of the same background strain (C57BL/6J; lane 1). Nonanemic mk/+ mice (lane 4) have more liver iron and express more hepcidin than anemic mk/mk homozygotes (lane 5), which have low iron stores and microcytic anemia. Homozygoushpx/hpx mice cannot efficiently deliver iron to developing red blood cells, resulting in severe anemia despite tissue iron overload (1594 μg iron per gram of liver). These mice have decreased hepcidin mRNA expression (lane 7) when compared with nonanemic littermates (lane 6). These results are representative of 2 experiments without significant variation between experiments.
Hepcidin mRNA expression is inappropriately high in adenoma tissue
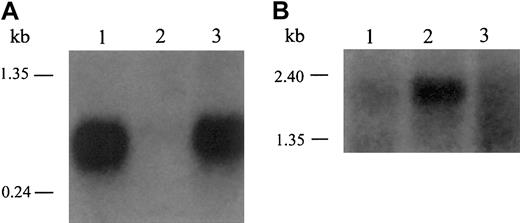
When we examined hepcidin mRNA expression in the human samples, we saw a dramatic increase in hepcidin mRNA in patient A's adenoma compared with her unaffected liver tissue (Figure3A). We compared these RNA samples with liver RNA from an anonymous, healthy, adult male subject who, based on his age, sex, and health, would be expected to have replete iron stores. We detected robust expression of hepcidin mRNA in the normal sample, indicating that hepcidin is usually expressed in appreciable amounts, as seen in wild-type, iron-replete mice (Figure 2). In contrast, patient A's unaffected liver tissue showed very little hepcidin mRNA expression, consistent with the fact that she has severe anemia. This observation is also consistent with the decreased expression of hepcidin mRNA in the livers of anemic mice (Figure 2). Importantly, although similar to the normal control, hepcidin mRNA expression in patient A's adenoma was 10 to 30 times that of her unaffected liver tissue. We conclude that the adenoma had failed to down-regulate hepcidin mRNA expression under the same conditions that suppressed hepcidin mRNA expression in the unaffected liver.
Inappropriate expression of hepcidin in adenoma tissue from GSD patients.
(A) Hepcidin is abundantly expressed in liver tissue from a healthy adult male (lane 1) but significantly decreased in the unaffected liver tissue from patient A (lane 2). In contrast, hepcidin mRNA expression in the adenoma tissue from patient A (lane 3) was considerably greater than the adjacent liver and similar to that of control liver. (B) We reprobed the same Northern blot for β-actin to control for RNA loading. These results are representative of 3 experiments without significant variation between experiments.
Inappropriate expression of hepcidin in adenoma tissue from GSD patients.
(A) Hepcidin is abundantly expressed in liver tissue from a healthy adult male (lane 1) but significantly decreased in the unaffected liver tissue from patient A (lane 2). In contrast, hepcidin mRNA expression in the adenoma tissue from patient A (lane 3) was considerably greater than the adjacent liver and similar to that of control liver. (B) We reprobed the same Northern blot for β-actin to control for RNA loading. These results are representative of 3 experiments without significant variation between experiments.
In situ hybridization analysis confirms differential hepcidin mRNA expression
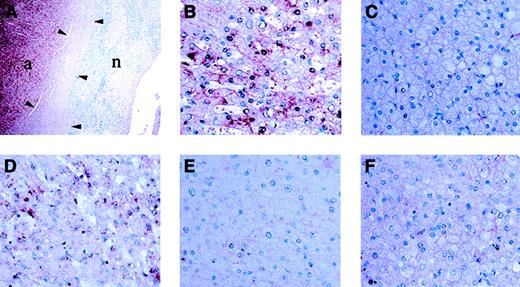
To determine whether this result was unique to patient A and her adenoma, we examined fixed tissue from a second GSD1a patient (patient B), who had undergone liver transplantation for complications of multiple, large adenomas. Similar to patient A, he suffered from microcytic anemia (hemoglobin [Hgb] level 8.5 g/dL or 5.3 mM; mean corpuscular volume [MCV] 64.0 fL) and decreased serum transferrin saturation (6.1%). He had prompt, spontaneous, postoperative resolution of his anemia. We subjected histologic sections from both patients to in situ hybridization (Figure4). Side-by-side comparison of signal intensity showed that hepcidin mRNA expression in unaffected liver tissue from both patients was significantly lower than in the adjacent adenoma tissue.
In situ hybridization analysis of hepcidin mRNA expression.
Hepcidin mRNA expression is low in unaffected liver tissue when compared with the adenoma tissue of patients A (A-C,F) and B (D,E). Adjacent adenoma tissue and unaffected liver tissue visualized at a magnification of × 10. (A) shows hepcidin mRNA expression is significantly greater in the adenoma. Adenoma tissue is indicated by “a,” and unaffected liver tissue is indicated by “n.” Arrowheads indicate the region occupied by the capsule at the interface between the liver and adenoma. Higher power (original magnification, × 100) confirmed that hepcidin mRNA expression is lower in hepatocytes of normal tissue (C,E) as compared with adjacent adenoma tissue (B,D) from the same tissue section. Hybridization with a hepcidin probe from the sense strand acts as a control for nonspecific staining (F). These results are representative of 2 experiments without significant variation between experiments.
In situ hybridization analysis of hepcidin mRNA expression.
Hepcidin mRNA expression is low in unaffected liver tissue when compared with the adenoma tissue of patients A (A-C,F) and B (D,E). Adjacent adenoma tissue and unaffected liver tissue visualized at a magnification of × 10. (A) shows hepcidin mRNA expression is significantly greater in the adenoma. Adenoma tissue is indicated by “a,” and unaffected liver tissue is indicated by “n.” Arrowheads indicate the region occupied by the capsule at the interface between the liver and adenoma. Higher power (original magnification, × 100) confirmed that hepcidin mRNA expression is lower in hepatocytes of normal tissue (C,E) as compared with adjacent adenoma tissue (B,D) from the same tissue section. Hybridization with a hepcidin probe from the sense strand acts as a control for nonspecific staining (F). These results are representative of 2 experiments without significant variation between experiments.
Discussion
Iron balance is primarily achieved through control of dietary iron uptake and distribution (reviewed by Andrews24). Erythropoiesis is highly dependent upon iron availability, and the most common nutritional cause of anemia is iron deficiency. Normally, most iron used for erythropoiesis is recovered from the degradation of senescent red blood cells by reticuloendothelial macrophages. When this recycling process is inefficient or macrophage iron release is inhibited, serum transferrin saturation falls and erythropoiesis is impaired.
Infection, malignancy, and chronic inflammation all may result in inefficient macrophage iron release and subnormal intestinal iron absorption, contributing to the anemia of chronic disease. These alterations have the effect of limiting the availability of iron to red blood cell precursors, even though total body iron stores may be adequate early in the course of the anemia. Some investigators have hypothesized that elevated cytokine levels induce changes in normal transfer of iron between macrophages and developing red blood cells,8 and some cytokines have been shown to alter the expression of macrophage transferrin receptor and ferritin,7 but there is currently no direct evidence that any particular cytokine inhibits cellular iron egress.
Hepcidin is an attractive candidate for a mediator of the anemia of chronic disease. It is exclusively produced in the liver and it circulates in plasma,10,11 consistent with its postulated role as a hormone involved in iron homeostasis. Further, hepcidin mRNA expression is increased in response to inflammatory stimuli such as lipopolysaccharide12 and infection.25 Although it has not yet been shown to interact with proteins of iron transport, its apparent activity12-14 suggests that hepcidin directly regulates the iron transport machinery. Studying mice with mutations affecting iron transport, we found that hepcidin mRNA expression is markedly decreased when erythropoiesis is iron limited, regardless of the status of iron stores.
Based on these results, we would have expected GSD1a patients with anemia to have very low hepcidin mRNA expression. We found that a patient with GSD1a produced very little hepcidin mRNA in her unaffected liver tissue, as expected, but her adenoma expressed high levels of hepcidin mRNA and did not respond appropriately to the signals that decreased hepcidin mRNA expression in the unaffected liver. Her anemia phenotype is remarkably similar to the severe anemia observed in transgenic mice that constitutively express hepcidin from a transgene but have down-regulated their endogenous hepcidin production.13 Accordingly, in our patient, there was a striking correlation between the event of adenoma resection and the correction of the anemia. We propose that the unregulated production of hepcidin by the adenoma impaired macrophage iron recycling and intestinal iron absorption, producing a clinical picture consistent with severe anemia of chronic disease. We do not yet know how adenoma hepcidin mRNA production escapes normal control mechanisms.
We believe that hepcidin plays a central role in the regulation of iron homeostasis and acts as a critical mediator in the anemia of chronic disease. Together, the observations that (1) hepcidin mRNA expression is increased in response to lipopolysaccharide12 and infection25 and (2) increased hepcidin mRNA expression results in severe anemia (shown here and by Nicolas et al13) suggest that hepcidin may be a downstream mediator of the iron recycling defect observed in individuals with the anemia of chronic disease. Elevated cytokines are hypothesized to participate in regulatory cascades that alter gene expression in developing erythroid cells and in the accessory cells of the bone marrow and spleen that influence red blood cell development and turnover.26Hepcidin may be one cytokine-regulated gene that impairs erythrocyte iron acquisition by modifying the iron egress activity of bone marrow and spleen macrophages. In addition to the block in iron recycling, elevated cytokines also result in decreased responsiveness of erythroid precursors to erythropoietin and decreased red blood cell survival,1 compounding the defect.
We do not yet know whether inappropriate hepcidin expression in the adenoma of GSD1a patients is mediated by cytokines or simply the direct result of tumor-specific changes in gene expression. However, when considered together, the increased severity of the anemia observed in GSD1a patients as compared with classical cases of anemia of chronic disease, the appropriately decreased expression of hepcidin in the unaffected liver of the 2 GSD1a patients presented here, and the direct relationship between tumor burden and the severity of the anemia in our cohort of GSD1a patients more strongly support the possibility that there have been tumor-specific changes in gene expression.
Unfortunately, no group has succeeded in producing antihepcidin antibodies thus far, so it is not yet feasible to measure hepcidin in clinical samples. However, measurement of hepcidin levels may be a useful diagnostic tool in the future. We do not yet know whether inappropriate hepcidin mRNA expression accounts entirely for the block in iron transport and distribution or whether other factors are also involved. Nonetheless, we speculate that our findings in GSD1a patients with hepatic adenomas will have broad implications for understanding the pathophysiology of abnormal iron distribution in patients with other chronic diseases. In patients with other diseases, endogenous liver hepcidin mRNA expression may be induced by inflammatory cytokines. If hepcidin is a central factor in this process, modulation of hepcidin bioactivity may offer new treatment strategies for patients with the anemia of chronic disease and other iron disorders.
We thank Kavita Sharma, Carolyn Starr, and Teresa Holm for technical assistance in the initial stages of this project and Drs John F. Crigler Jr and Ellis Neufeld for sharing their clinical insights into the anemia of patients with GSD1a. We thank Dr Hiromi Gunshin for sharing related data prior to publication. Yu Yang and Tamas Onody helped with the in situ hybridization experiments in the In Situ Hybridization Core Facility at the Dana-Farber Cancer Institute.
N.C.A. is an associate investigator of the Howard Hughes Medical Institute.
Prepublished online as Blood First Edition Paper, June 28, 2002; DOI 10.1182/blood-2002-04-1260.
D.A.W. and C.N.R. contributed equally to this work.
Supported in general by a Clinical Research Center Grant from the Public Health Service Division of Research Resources (NIH M01RR02172), a grant from the Association for Glycogen Storage Disease (USA), and the Children's Hospital Glycogen Storage Disease Research Fund. D.A.W. was supported by the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center-Harvard/Massachusetts Institute of Technology (MIT) Health Sciences and Technology in collaboration with Pfizer. C.N.R. is supported by the Hematology Training Grant T32-HL07623-15. M.D.F. is supported in part by National Institutes of Health (NIH) Grant K08-03600.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nancy C. Andrews, Division of Hematology/Oncology, Enders 720, Children's Hospital Boston, 300 Longwood Ave, Boston, MA 02115; e-mail:nandrews@enders.tch.harvard.edu.