After myeloablative treatment and allogeneic stem cell transplantation (SCT), patients are kept in isolation rooms in the hospital to prevent neutropenic infections. During a 3-year period, patients were given the option of treatment at home after SCT. Daily visits by an experienced nurse and daily phone calls from a physician from the unit were included in the protocol. We compared 36 patients who wished to be treated at home with 18 patients who chose hospital care (control group 1). A matched control group of 36 patients treated in the hospital served as control group 2. All home care patients had hematologic malignancies and 19 were in first remission or first chronic phase. Of the donors, 25 were unrelated. The patients spent a median of 16 days at home (range, 0-26 days). Before discharge to the outpatient clinic after SCT, patients spent a median of 4 days (range, 0-39 days) in the hospital. In the multivariate analysis, the home care patients were discharged earlier (relative risk [RR] 0.33, P = .03), had fewer days on total parenteral nutrition (RR 0.24, P < .01), less acute graft-versus-host disease (GVHD) grades II-IV (RR 0.25,P = .01), lower transplantation-related mortality rates (RR 0.22, P = .04), and lower costs (RR 0.37, P < .05), compared with the controls treated in the hospital. The 2-year survival rates were 70% in the home care group versus 51% and 57% (not significant) in the 2 control groups, respectively (P < .03). To conclude, home care after SCT is a novel and safe approach. This study found it to be advantageous, compared with hospital care.
Introduction
Over the past 30 years, allogeneic hematopoietic stem cell transplantation (SCT) has emerged as a curative therapy for a number of lethal disorders affecting the hematopoietic system.1-5 After conditioning with high doses of chemoradiotherapy, pancytopenia occurs and infectious complications, including bacterial bacteremia, invasive fungal infections, and viral infections, are common.6-9 During the pancytopenic phase, the patients are kept in a protected environment, such as laminar air flow rooms (LAF) or reversed isolation.10-13 Despite this, infectious complications are common causes of morbidity and mortality shortly after SCT.
Home care is mainly used for palliative care in end-stage cancer patients and in geriatrics.14,15 However, there is some controversy regarding the quality of life and costs of home care as compared with those of hospital care.16-19 In more recent years, home care for outpatients has been given in some centers performing autologous SCT.15 One study of SCT allowed patients to leave their rooms and the hospital at will.20Patients living close to the hospital were allowed to go home for a few hours and sometimes overnight. We used another approach in our patients who underwent SCT. After conditioning and transplantation in the hospital, the patients were given the opportunity to be treated at home during the pancytopenic phase. An experienced nurse from our unit visited the patients once or twice daily until the patient could be discharged to the outpatient clinic. To our knowledge, this has not been done before. A pilot study including 11 patients treated at home in this way showed that the procedure was safe.21 In the present study, we planned to treat 36 patients at home during the pancytopenic phase; 2 of them could not go home for medical reasons. We compared these 36 patients with 18 patients who were offered home care, but preferred hospital care. Since some of these patients might have been less psychologically fit than those treated at home, we also compared a second control group of 36 patients, matched for various risk factors, with the home care group. They came from other parts of Sweden, or abroad, where home care was not possible, because they lived too far from the hospital. The aim of the study was to compare outcome of home care with hospital care after SCT.
Patients, materials, and methods
Patients and patient selection for home care
From March 1, 1998, until December 31, 2000, 179 patients underwent allogenic SCT at Huddinge University Hospital and of those 60 lived in the Stockholm area. All patients living within one hour's driving distance from Huddinge University Hospital undergoing SCT and judged eligible by our medical team were offered home care from the day of transplantation. There were 6 of the 60 patients living in the Stockholm area who were not asked because they didn't speak Swedish, were addicted to narcotics, or were considered medically and psychologically unfit.2 Of the 54 patients who were asked, 36 preferred to stay at home and fulfilled the following requirements: (1) a caregiver (relative or friend) was willing to stay at home and help; and (2) approval of the home by the head of the Department of Infection Control. The department required that the water temperature must be more than 50°C to prevent the spread by tap water of legionnaires' disease, there be no flowers in pots because the earth may contain Aspergillus, there be no pet animals at home, the sheets be changed 3 times a week, and that the home be cleaned once a week. These criteria could not be fulfilled by 18 patients who either had no caregiver,15 had pets in the home,2 or would not feel safe at home,1 and they served as control group 1. None of these patients were excluded because of their clinical condition. To avoid the bias that more fit and determined patients chose to stay at home than those treated in the hospital, we also selected a control group of patients not eligible for the study, because they resided outside the Stockholm area and matched them for as many variables as possible including diagnosis, stage of disease, age, sex, type of donor (related, unrelated), source of stem cells (bone marrow [BM] or peripheral blood stem cells [PBSCs]), and conditioning (Table 2). Of the 36 patients in control group 2, 23 underwent allogenic SCT between March 1, 1998, and December 31, 2000, and 13 patients underwent allogenic SCT either before or after this period. From March 1, 1998, until December 31, 2000, among the 179 patients, 75 were included in the study (plus 2 who received retransplants), 30 patients had hematologic malignancies but could not be well matched for prognostic variables with the home care patients, 46 were children younger than 18 years of age, 20 had solid tumors, 2 had aplastic anemia, one had a metabolic disorder, 4 received “minitransplants,” and one received an HLA antigen–mismatched graft. There were 2 patients in the home care group who could not go home after the transplantation as planned, because they were in too poor clinical condition. One had retinitis and was almost blind and the other was admitted to the intensive care unit (ICU) because of respiratory insufficiency and multiorgan failure. Both patients were included in the home care group so as not to introduce a bias and because we wished to treat them at home. The study group and the controls were well matched for diagnosis, disease status, sex, age, type and age of donor, source of stem cells, granulocyte–colony-stimulating factor (G-CSF) treatment after the transplantation, and conditioning (Table1). Control group 2 showed a trend for a lower median dose of nucleated cells than the study group (P < .06). The study was approved by the ethics committee at Huddinge Hospital, Karolinska Institutet. Informed consent was provided according to the Declaration of Helsinki.
Information
All patients and their caregivers (a relative or friend) were informed about the procedure before they chose home care or hospital care. Conditioning was given in the hospital and the patient and caregiver received information and education about the procedure by the staff, social workers, dietician, and physical therapist. The caregiver stayed together with the patient in the hospital during this time to learn about the procedure and to know the staff. The most important thing for the caregiver at home was to be company for the patient, to make food if needed, and to give a call to the hospital if help was necessary. We wished to give the patient an opportunity to stay at home as much as possible if he/she wanted to. The patients were always welcome back at the hospital if they or the caregiver preferred it. We did not have an empty bed waiting for the patients who chose home care, but we had planned in advance which room could be used in case a home care patient came to the hospital.
Conditioning
Conditioning consisted of 60 mg/kg cyclophosphamide (Cy) for 2 days, combined with 10 Gy of total body irradiation (TBI), single fraction, with the lungs shielded to receive no more than 9 Gy, or fractionated 3 Gy daily for 4 days.22 The amount of 4 mg/kg per day busulfan (Bu), divided into 4 doses given for 4 days (total dose 16 mg/kg), was adjusted to the Bu levels.23,24It was combined with 60 mg/kg Cy for 2 days. A few patients were given reduced conditioning including 30 mg/m2 per day fludarabine for 6 days, combined with 4 mg/kg per day Bu for 2 days (total dose 8 mg/kg), combined with 2 mg/kg per day thymoglobulin (Sangstat, IMTIX, Lyons, France) for 4 days.25 In patients who received unrelated grafts, 2 mg/kg per day thymoglobulin was given for 2 to 4 days before SCT.22 26
Centre infrastructure and outpatient management
The Centre for Allogeneic Stem Cell Transplantation (CAST) has 12 single rooms, 6 with reversed isolation. There are 6 doctors, 15 nurses, and 10 assistant nurses. Every day we have at least 4 doctors, 4 nurses, and 3 assistant nurses at the ward and one nurse for home care during daytime. During nights we have one doctor on call and 2 nurses in service at the ward. We have one senior doctor on call at home. After discharge from CAST, adult patients are referred to the outpatient clinic at the Department of Hematology and children to the Department of Pediatric Hemato-oncology. At the outpatient clinic, check-ups are performed twice weekly for the first 3 months and thereafter less frequently, dependent on the status of the patient. When patients are readmitted to the hospital, adults are cared for at CAST and children at the Department of Pediatrics.
Home care
After the graft had been infused, the patients could go home. An experienced nurse from the ward visited the patient once or twice daily, for a median of 1 hour (range, 0.5 to 3 hours), depending on the needs of the patient. The nurse checked vital signs, including temperature and blood pressure, and examined the patient's mouth for mucositis, herpes lesions, and fungi, as well as the skin for acute graft-versus-host disease (GVHD) or other lesions. In the morning, the nurse took blood samples from the central venous line (10-25 mL/d) and gave intravenous medications, erythrocyte transfusions if the patient had a hemoglobin (Hb) level less than 80 g/L, and platelet transfusions when the platelet count fell below 30 × 109/L, or if there were signs of hemorrhage.21-23 26 If the patient's fluid intake was less than 2 liters in 24 hours and weight had decreased by more than 2 kg, parenteral nutrition was started. If the patient could not feed himself or herself at all, total parenteral nutrition (TPN) was given. Mucosal pain was treated with oral paracetamol or oral morphine. If this was not sufficient, continuous intravenous morphine was given, using a home pump. If the nurse needed any advice, she called the physician at the ward when she visited the patient. At the hospital, the nurse and the physician went through all the clinical and laboratory data. After this, the physician called the patient to tell him or her about the chemistry results, to check the patient's status, and to change medications, if needed. The patient was asked to take his or her temperature frequently and if it rose above 38.5°C, the patient was to call the unit and return to the hospital. This was done to ensure that the patient did not develop septic shock or acute respiratory distress syndrome at home. Blood cultures and a chest x-ray were taken at the SCT unit and intravenous antibiotics were started. The patient received a check-up in the hospital for an infection and, if the patient felt well, he could go home even with a fever; intravenous antibiotics were continued at home. Criteria for admission to the ward were (1) deterioration of the patient's condition, (2) if the patient's temperature rose above 38.5°C, at least twice, (3) if the patient needed intravenous injections more than twice daily, and (4) if the caregiver was unable to stay at home and support the patient. Before admission to the ward, the patient or the caregiver always contacted the responsible physician.
Hospital care
Patients being cared for at the hospital were treated in conventional single rooms with reversed isolation and a relative or friend could stay with them.22 They could take a walk outside the hospital after 6:00 pm on weekdays and during weekends.21,22 26 Patients treated in the hospital and at home were asked to avoid persons with symptoms of or having contagious diseases, going near construction areas when they were out walking due to the risk of aspergillosis, visiting anyone, and shopping. Patients in the hospital participated in a prospective randomized trial comparing platelet transfusions when platelet counts fell less than 30 × 109/L versus less than 10 × 109/L.
Infection prophylaxis
Infection prophylaxis was the same for the home care patients as for those treated in the hospital. During conditioning, all patients started with gut decontamination consisting of 500 mg oral ciprofloxacin twice a day and 250 mg amphotericin B once a day until neutrophils were more than 0.5 × 109/L. Co-trimoxazole was given as prophylaxis against Pneumocystis carinii during conditioning until 2 days before transplantation and after SCT when the absolute neutrophil count (ANC) was more than 0.5 × 109/L and was maintained for 6 months. To avoid myelotoxicity induced by co-trimoxazole, 15 mg leukovorin was given intravenously once daily until ANC was more than 0.5 × 109/L. Leukovorin was not given the day before or on the day when methotrexate injections were given. To prevent oral candidiasis, mycostatin was given from the day of SCT once a day for the first 3 months after transplantation. Patients with a herpes simplex virus immunoglobulin G (IgG) titer of more than 10 000 (determined by enzyme-linked immunosorbent assay [ELISA]) received oral or intravenous acyclovir prophylaxis until ANC was more than 0.5 × 109/L. Granulocyte colony-stimulating factor at 5 μg/kg per day was given from day +10 after SCT until ANC was more than 0.5 × 109/L for 2 consecutive days.
Blood cultures were taken the first time the patient had a temperature of more than 38.5°C and cultures from urine, nasopharynx, stool, or the central venous line incision were taken, when indicated. Subsequent blood cultures were obtained when patients had a high fever and chills or in the event of a continuous fever, 2 to 3 times weekly.
Immunosuppression and donors
Cyclosporine (CyA) combined with 4 doses of methotrexate was given as prophylaxis against GVHD.22,23,26,27 One patient in the home care group with a twin donor received no prophylaxis. Only a few patients were given CyA and prednisolone (Table 1). Of the donors in the home care group, one was an identical twin, 10 were HLA antigen–identical siblings, and 25 were HLA-A–, HLA-B–, and HLA-DRβ1–compatible unrelated. HLA antigen matching criteria were the same for the home care and the hospital care patients. In control group 1, 7 donors were HLA antigen–identical siblings and 11 were unrelated donors. In control group 2, 12 donors were HLA antigen–identical siblings and 24 were unrelated donors. Details regarding treatment have been reported elsewhere in detail.22,23 26
Monitoring
Patients at home were monitored using the same charts and chemistry as patients in the hospital. Patient therapy compliance was noted in the charts.
Statistics
The Fisher exact test was used to compare the distribution of patients with bacteremia and the Mann-Whitney U test to compare days with fever, TPN, antibiotics, transfusions, and other data. The probability of GVHD, transplantation-related mortality (TRM), relapse, leukemia-free survival (LFS), and survival rates were compared using the method of Kaplan-Meier with the log-rank test (Mantel-Haenszel).28 Cox regression model was used for the multivariate analysis.29 Factors withP = .1 in the univariate analysis were included in the multivariate analysis. The following factors were analyzed: home care or hospital care, type of donor (sibling/unrelated), source of stem cells (BM vs PBSC), diagnosis, stage of disease (early was defined as first remission or chronic phase; late was defined as more advanced), sex, age, cytomegalovirus (CMV) serology, fever, bacteremia, acute GVHD (grade 0-I vs grade II-IV), time to engraftment, absolute neutrophil count (ANC more than 0.5 × 109/L), nucleated cell dose, donor age, donor sex, and female donor to male recipient. Home care was the main factor to be tested whereas all the other factors were included to control for differences between the groups. To correct for multiple comparisons a Bonferroni correction was made. As 5 multivariate analysis were made, the new significance level will be .05/5 = .01. Only patients surviving more than 30 days were included in the analysis of acute GVHD. A minimum of 90 days of follow-up was a criterion for relapse and chronic GVHD.
Calculation of costs
The costs were calculated from the day of transplantation. The calculation did not include costs prior to transplantation, such as tissue typing, donor search, cell harvest, and so forth. The costs, calculated in US dollars as $1084 per day in the hospital, included medication, hospital bed, and staff. The same costs were calculated for patients staying at home, because the ward also served as a back-up for the home care patients. The costs were calculated until day 76, that is, the last day of discharge from the hospital among the control patients (Table 2). Every visit to the outpatient clinic was estimated at $200. The cost per day when patients were readmitted to the ward was $798. This calculation does not include loss of income to patients and caregivers. Such costs are covered by the health insurance system in Sweden. Patients in the hospital could also have a relative or a friend staying with them. The health care system in Sweden pays for a relative for 60 days as a caregiver.
Results
Days at home in the home care group
Among the 36 patients in the home care group, 2 never went home because they were too sick to leave the hospital. The others went home on median day +1 after the transplantation (range, 0-8 days). One patient had to wait until day 8 because she had no caregiver at home until then. Of the 34 patients who went home, 21 were readmitted to the ward on 33 occasions, median 1 day (range, 0-25 days), because of fever (n = 24), no caregiver at home (n = 2), diarrhea and/or fever and/or pain (n = 3), pain (n = 1), GVHD (n = 1), nausea and vomiting (n = 1), and mucositis (n = 1).
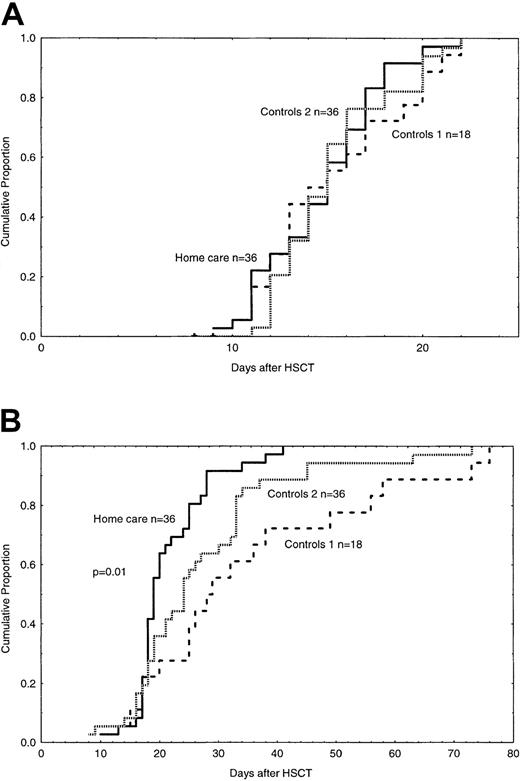
The time to discharge to the outpatient clinic was significantly faster in the home care group than in the control groups—that is, median 19 days versus 29 days (Table 3,P < .01). In the univariate analysis, a short time to discharge was associated with home care, fast engraftment, no CMV infection, and no bacteremia (Table 4, Figure 1). In the multivariate analysis, fast engraftment, home care, and no CMV reactivation were associated with a short time to discharge (Table 5).
Engraftment of ANC and time to discharge.
(A) Time to and cumulative incidence of ANC of more than 0.5 × 109/L in patients treated at home (solid line) (ns) and in control group 1 (large dashed line) and control group 2 (small dashed line). n indicates number of patients in each group. (B) Time to and cumulative incidence of discharge to the outpatient clinic in patients treated at home (solid line) (P < .01) or in control group 1 (large dashed line) and control group 2 (small dashed line).
Engraftment of ANC and time to discharge.
(A) Time to and cumulative incidence of ANC of more than 0.5 × 109/L in patients treated at home (solid line) (ns) and in control group 1 (large dashed line) and control group 2 (small dashed line). n indicates number of patients in each group. (B) Time to and cumulative incidence of discharge to the outpatient clinic in patients treated at home (solid line) (P < .01) or in control group 1 (large dashed line) and control group 2 (small dashed line).
Fever and infections
We found no difference in the number of days with fever of 38.5°C or higher in the home care group versus the control groups (Table 3), but, significantly more blood cultures were taken in the 2 control groups than in the home care group (P = .01). Bacteremia occurred in 25% of patients in the home care group versus 44% and 39% in the 2 control groups, respectively (Table 3, ns). In the multivariate analysis, bacteremia was associated with a male recipient (P < .05). The number of days on intravenous antibiotics was the same in the 3 groups (Table 3).
Engraftment and transfusions
Engraftment, time to a white blood cell (WBC) count of more than 0.2 × 109/L, time to an ANC of more than 0.5 × 109/L, and time to a platelet count of more than 30 × 109/L were similar in the home care group and the control groups (Table 3, Figure 1A). Likewise, we found no difference in number of platelet transfusions in the home care group and the controls. However, control group 1 needed significantly more erythrocyte transfusions (median 7 vs 4) than the home care group (Table 3, P < .05).
Analgesics and TPN
A median of 1 day on analgesics in the home care group was significantly lower than a median of 15 in control group 1 (Table 3,P < .05). In the multivariate analysis, intravenous analgesics were associated with CMV seropositivity in the recipient and/or donor (Table 5). A median of 4 days on TPN in the home care group was significantly shorter than 23 and 10 days in the control groups 1 and 2, respectively (Table 3, P < .001,P < .01). In the univariate analysis, days on TPN was associated with hospital care, delayed engraftment, CMV reactivation, and bacteremia (Table 4). In the multivariate analysis, TPN was associated with hospital care and delayed engraftment (Table5).
GVHD and TRM
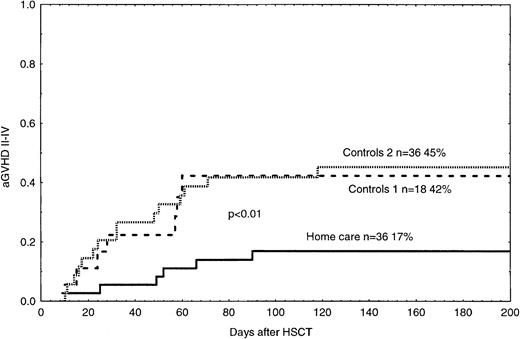
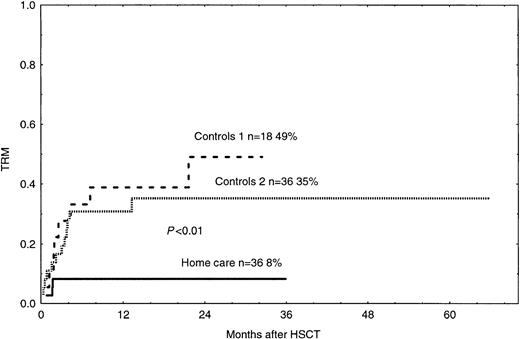
The probability of grades II-IV acute GVHD in the home care group was 17%, which was significantly lower than 42% and 45% in the control groups 1 and 2, respectively (Figure2, P < .05). In the univariate and multivariate analyses, acute GVHD grades II-IV was associated with hospital care and PBSCs, as compared with BM as the cell source (Tables 4 and 5). TRM was 8% in the home care group, which was significantly better than 49% and 35% in the control groups 1 and 2, respectively (Figure 3,P < .01, P = .02). In the univariate and multivariate analysis, TRM was associated with acute GVHD, hospital care, and bacteremia (Tables 4 and 5).
Time to and cumulative incidence of acute GVHD grades II-IV in home care patients (P < .05) and control groups 1 and 2.
Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2.
Time to and cumulative incidence of acute GVHD grades II-IV in home care patients (P < .05) and control groups 1 and 2.
Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2.
Time to and cumulative incidence of TRM in home care patients (P < .01) and control groups 1 and 2.
Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2.
Time to and cumulative incidence of TRM in home care patients (P < .01) and control groups 1 and 2.
Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2.
Reasons for death and survival
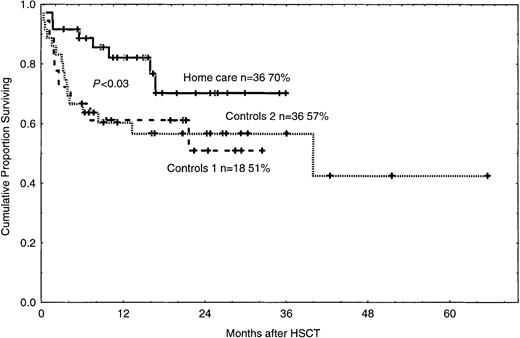
The reasons for death in the 3 groups are listed in Table6. The 2-year survival rate was 70% in the home care group (P < .03), compared with 51% and 57% in control groups 1 and 2 (Figure4). In the univariate analysis, death was associated with acute GVHD grades II-IV, bacteremia, and hospital care (Table 3). In the multivariate analysis, death was associated with GVHD and bacteremia (Table 4).
Cumulative proportion of surviving patients in the home care group (P < .03) and in control groups 1 and 2.
n = number of patients in each group. The figure shows 2-year survival. Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2. Each plus sign indicates a surviving patient.
Cumulative proportion of surviving patients in the home care group (P < .03) and in control groups 1 and 2.
n = number of patients in each group. The figure shows 2-year survival. Solid line indicates home care patients; large dashed line, control group 1; small dashed line, control group 2. Each plus sign indicates a surviving patient.
Bonferroni correction
After correction for multiple analysis (Bonferroni correction), home care was statistically associated with fewer days on TPN (P < .01) and a lower incidence of acute GVHD grades II-IV (P = .01) (Table 5).
Quality of life
All patients and their caregivers answered an anonymous questionnaire when they were discharged to the outpatient clinic. No patient treated at home regretted this decision. They were glad to stay with their families and to take part in activities at home and walk when they felt like it. One patient given reduced conditioning and treated at home was a 57-year-old lawyer with myelodysplastic syndrome (MDS). He took a walk to and spent a couple of hours at his work every day. A 54-year-old woman with AML M4, treated with complete myeloablative therapy, got up every morning at 6:00 am when the nurse arrived and invited her to coffee. She made her bed every day and did the laundry to get some exercise. A 15-year-old boy with AML M4 having car sickness declined to visit the hospital to see the physician who wished to examine his blisters. The physician therefore went to his home instead.
Costs
The median cost of treatment from day 0 until day +76 was $25 340 for the home care group, compared with $36 437 for control group 1 (P < .001) and $33 620 for control group 2 (P < .05, Table 2). In the multivariate analyses, high costs were associated with late engraftment, acute GVHD grades II-IV, and hospital care (Table 5).
Discussion
This study of home care during the pancytopenic phase for patients who underwent SCT used experienced nurses from the Stem Cell Transplant Ward and was supported by a grant from the Swedish Cancer Society for 3 years. The main reason for the project when it started was to allow the patient to be treated at home instead of in a hospital. The first aim was to find out whether home care was safe and useful for the patients and their relatives. The trial was not randomized because we wished to treat as many patients as possible at home during this period. Many people opposed this because they thought the patients might die at home. Therefore, the pediatricians initially refused to have children participate in the study; only adults were included. To reduce the risk of sudden death at home (eg, from septic shock or the adult respiratory distress syndrome), patients were taken to the hospital when they had a fever of more than 38.5°C. Many of them (62%) were readmitted to the unit. However, after a median of 1 day in the hospital, they could go home again and and stay there.
The first patients and their caregivers were very enthusiastic and, after 17 months, 11 patients had been treated at home. We then did a safety evaluation of the study.21 At that time, 22 patients had been given the choice of being treated at home. There were 11 patients who could not be treated at home, and they served as controls. In this evaluation, we found to our surprise that the patients treated at home had bacteremia less often, spent fewer days on TPN, had fewer erythrocyte transfusions, and had fewer days on intravenous antibiotics and intravenous analgesics than the controls. This preliminary study indicated not only that it was safe to be treated at home during the neutropenic phase after SCT, but better in many respects than isolation in the hospital.
As the study continued, more patients wanted to be treated at home and 2 children, 14 and 15 years of age, were included. Although 36 patients had agreed to be treated at home, 18 were not because they had no caregiver, felt safer in the hospital, and so forth. We were criticized for selecting controls who were eligible for the home care project but who could not take part in it. This could have introduced a bias in which the controls treated in the hospital were more or less psychologically fit and had a worse outcome than those treated at home. To overcome this problem, we added another control group consisting of 36 patients who could not be offered treatment at home because they lived too far away from the Stockholm area. These controls were matched for as many prognostic variables as possible (Table 1). On the whole, they are typical of those who have undergone a transplantation in our unit during the past few years. For instance, we use more unrelated donors than HLA antigen–identical siblings and PBSCs more frequently than BM.26 30
The home care group had several advantages compared with the 2 control groups. They could be discharged to the outpatient clinic faster although the times to engraftment of ANC and platelets were the same (Table 3). Since the home care patients usually took care of their food and medication themselves, they could be discharged to the outpatient clinic earlier than those treated in the hospital who more often had trouble in eating. Other reasons for an earlier discharge may be that the home care patients could eat and drink more and therefore required less TPN than the controls treated in the hospital, and were more active and therefore had a better appetite. They could also go to their own kitchen whenever they wanted and take something they were used to and liked to eat. They probably felt more like eating because they could eat their meals together with their families. The larger space and the walks outside may also have stimulated the appetite. They also may have forced themselves to eat because of the wish to stay at home.
We also found that the home care patients were less likely to develop grades II-IV acute GVHD than the controls (Figure 2, Tables 4 and 5). The reasons for this may have been better nutrition and maybe a trend for less bacteremia. Infections can lead to GVHD. For instance, gnotobiotic mice have a lower risk of developing GVHD.31,32 A clinical study showed that patients treated in laminar air flow rooms were less likely to develop GVHD than those treated in regular hospital rooms.33 Because of the lower risk of GVHD in the home care group, TRM was also significantly lower in this group than in the controls (Figure 3, Tables 4 and 5).
In the safety analysis of this study, the main concern was the risk of septic shock or the adult respiratory distress syndrome, which are fatal complications. However, none of the patients in the 3 groups died of these conditions. Another concern was whether the risk of anAspergillus infection would increase in the home care patients, who were not isolated. However, so far, no patient has acquired a clinical Aspergillus infection. Indeed, such infections were rare in our patients who underwent SCT.7This may be due to our cool climate, since other studies have reported reduced Aspergillus infection rates in patients who underwent SCT who are strictly isolated.12 34
In the analysis of TRM and survival, the disease and stage of the disease were not significant in comparison with GVHD, bacteremia, and hospital care. One reason for this may have been the short follow-up because only a few patients so far have had a relapse of their hematologic malignancy (Table 6). Relapse is otherwise a major cause of mortality after SCT for hematologic malignancies.1-5
It is obvious that most patients who were given the opportunity to be treated at home appreciated this option. Unfortunately, this could only be offered to those living close to the hospital and a specialized SCT unit. Indeed, no patient regretted this decision. As regards the quality of life, we could not compare the groups, because most of those treated in the hospital were not eligible for treatment at home.
As regards costs, it was cheaper to be cared for at home because home care patients were discharged earlier to the outpatient clinic (Tables2, 3, and 4). Home care is probably also cheaper than hospital care because home care patients required less TPN and antibiotics and, in addition, TRM was reduced and more lives were saved. Furthermore, fewer nurses and doctors were needed and the hospital beds could be used more efficiently. Home care can function only if experienced nurses from an SCT unit and hospital beds are available when needed in case of an emergency or high fever. Then, home care can be used to supplement hospital care for patients living near specialized SCT units.
There are several differences favoring the home care arm. With the Bonferroni correction, it cannot be excluded that the lower TRM may be influenced by chance. Still, the study provides evidence that patients are not put at risk by being treated at home.
To conclude, home care during the pancytopenic phase after SCT is a novel and safe approach. According to this study, home care had several advantages (eg, faster discharge, reduced need for TPN, a lower incidence of acute GVHD, lower TRM, and lower costs) over treatment in the hospital. This study should be used as the basis for a prospective randomized study comparing home care with hospital care after SCT.
We thank the caregivers and the patients who participated in this study. We also thank all nurses (especially Birgitta Bjurman), physicians, and other staff who helped in this study; Inger Hammarberg for secretarial help; Bengt Jönsson, professor of Health Economics, Stockholm School of Economics, for advice regarding evaluation of costs; and Bo Nilsson, BSc, Department of Cancer Epidemiology, Karolinska Institutet, for statistical advice; and editor Francis Walsh and Dr Zoe Walsh for checking the language. This article is dedicated to Dr Karl-Erik Myrbäck, former head of the Department of Hospital Control, Huddinge University Hospital, for his belief in this project when so many were against it. He was an enthusiastic coworker in this project, but died tragically before it was finished.
Prepublished online as Blood First Edition Paper, August 22, 2002; DOI 10.1182/blood-2002-03-0801.
Supported by grants from the Swedish Cancer Society (999508 and 0070-B99-13XAC), the Children's Cancer Foundation (1997/073), the Swedish Medical Research Council (K2000-06X-05971-20A), the Cancer Society in Stockholm, the Tobias Foundation, the FRF Foundation, and Karolinska Institutet.
Karl-Erik Myrbäck died on June 19, 2001.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Britt-Marie Svahn, Ward Director, Centre for Allogeneic Stem Cell Transplantation, Huddinge University Hospital, B87, SE-141 86 Stockholm, Sweden; e-mail:b-m.svahn@transpl.hs.sll.se.