Increased apoptosis of hematopoietic progenitor cells has been implicated in the pathophysiology of cytopenias associated with myelodysplastic syndromes (MDSs), and inhibition by immunosuppression may account for the success of this treatment in some patients. We examined bone marrow and peripheral blood of 25 patients with chromosomal abnormalities associated with MDS (monosomy 7, trisomy 8, and 5q−) for evidence of apoptosis. When fresh bone marrow was examined, the number of apoptotic and Fas-expressing CD34 cells was increased in patients with trisomy 8, but decreased in monosomy 7, as compared with healthy control donor marrow. Fas expression was increased in the trisomy 8 cells and decreased in the monosomy 7 cells when compared with normal cells from the same patient. Trisomy 8 cells were more likely to express activated caspase-3 than were normal cells. For bone marrow cells cultured with Fas agonist or Fas antagonist, the percentage of cells with trisomy 8 was significantly decreased in most cases after Fas receptor triggering and increased by Fas ligand (Fas-L) antagonist (P < 0.01), suggesting increased Fas susceptibility of cells with trisomy 8. No such changes were seen in cultures of cells with 5q− or monosomy 7. Fas antagonist facilitated the expansion of cells with trisomy 8 only. Cells with trisomy 8 appear to be more susceptible to Fas-mediated apoptosis. Clinical data demonstrating the responsiveness of some patients with trisomy 8 to anti–thymocyte globulin (ATG) and cyclosporine (CsA) would favor an active role of the immune system in this syndrome.
Introduction
Myelodysplastic syndromes (MDSs) are a heterogeneous group of disorders associated with clonal proliferation of hematopoietic cells, bone marrow failure, and transformation to acute leukemia. Excessive apoptosis of both progenitor and mature cells occurs in some patient groups, often with up-regulation of Fas receptor (Fas-R) and Fas ligand (Fas-L) expression. Although many initial studies were performed on the whole marrow cell population,1-3 purified CD34 cells in some cases show increased apoptotic cell markers.4 Conversely, some investigators have demonstrated that CD34 cells from patients with MDS show resistance to apoptosis.5 However, this discrepancy may be related to patient selection; the numbers of apoptotic CD34 cells appeared to inversely correlate with the prognostic stage, and patients with evidence of increased apoptosis may have better outcomes.4,6 One study demonstrated that CD34 cells from patients with monosomy 7 were resistant to apoptosis compared with CD34 cells from patients with other cytogenetic abnormalities.4
Resolution of cytopenias after treatment with immunosuppressive therapies such as anti–thymocyte globulin (ATG) and cyclosporine suggests that immunologic mechanisms are important in the pathophysiology of some MDS-related bone marrow suppression.7 The cytogenetic abnormalities that are frequent in MDS could be the primary event, leading to the functional abnormalities observed, including an immune response to new antigens expressed by clones of aberrant cells. Alternatively, cells with chromosomal abnormalities might arise secondarily, in a damaged bone marrow characterized by a high rate of cell death, rapid cell turnover, and telomere shortening, through some mechanism of genomic instability.8 9 Immunosuppressive therapies might well be effective in either scenario.
In the current work, we have examined the sensitivity to apoptosis of cells with trisomy 8, monosomy 7, and 5q− common cytogenetic abnormalities in MDS, in an attempt to explain differences in clinical behavior among patients with these syndromes.
Patients, materials, and methods
Cell preparation
Bone marrow (BM) samples were obtained from patients with MDS by French American British (FAB) criteria and from healthy volunteers after informed consent according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute. BM was obtained by aspiration from the posterior iliac crest into syringes containing media supplemented 1:10 with heparin (O'Neill and Feldman, St Louis, MO). BM mononuclear cells (BMMNCs) were isolated by density gradient centrifugation with the use of lymphocyte separation medium (Organon, Durham, NC).
Fluorescent in situ hybridization
At days 0, 7, and 14 of culture, cells were treated with hypotonic buffer consisting of KCL,N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), ethyleneglycotetraacetic acid (EGTA), and NaOH to expose the nucleus at interphase and fixed onto slides using methanol and acetic acid (3:1). Fluorescent in situ hybridization (FISH) was performed with probes for chromosomes 5q, 7, and 8 (Vysis, Downers Grove, IL). Slides were denatured by immersion in formamide/20 × standard saline citrate (SSC) solution for 5 minutes at 73°C followed by several washes in 70%, 85%, and 100% ethanol at room temperature for 1 minute each. Probes were denatured as above and applied to the cells on the slides to hybridize at 42°C overnight. After hybridization, the slides were washed in prewarmed 0.4 × SSC at 73°C for 2 minutes, and 2 × SSC/0.1% Nonidet P-40 (NP-40) at room temperature for 1 minute. The slides were allowed to dry in the dark and were counterstained with DAPI-II (4,6-diamidino-2-phenylindole–II) for enumeration by means of a fluorescence microscope. The percentage of positive staining was based on 200 cells scored.
Immunohistochemical detection of activated caspase-3
BM smears were allowed to air-dry overnight. Without fixation, slides were incubated with FAM-DEVD–fluoromethyl ketone (FAM-DEVD-FMK), the carboxyfluorescein analog ofN-benzyloxycarbonyl–DEVD-FMK (zDEVD-FMK), diluted with dimethyl sulfoxide (DMSO) to 150 × concentration and then further diluted with 1 × phosphate-buffered saline (PBS) to the final 30 × working dilution. The incubation time was 1 hour at 37°C. After washing for 5 minutes with 1 × washing buffer (Caspa Tag Kit; Intergen, Purchase, NY), the slides were counterstained with Hoechst dye for 5 minutes at 37°C and washed again, then fixed in acetone for 10 minutes, followed by ice-cold Carnoy fixative for 10 minutes. and 1% paraformaldehyde in 2 × SSC for 1 minute. After dehydration in 70%, 85%, and 100% ethanol for 1 minute each, FISH was performed as described above.
Flow cytometric separation of CD34 cells and cells expressing Fas
For specific analysis of purified populations of cells expressing Fas and CD34, cells were stained with phycoerythrin (PE)–conjugated monoclonal antibodies directed at CD34 or Fas, washed with PBS, and sorted by microcytofluoremetry (Epics V; Coulter, Miami, FL).
Long-term bone marrow cultures to determine if cytogentic abnormalities are present in long-term colony-initiating cells
To analyze the most immature progenitor and stem cell compartment, we used long-term bone marrow culture (LTBMC); both stroma cell culture and long-term colony-initiating cell (LTCIC) assay were performed. For stromal culture, 10 × 106allogeneic BMMNCs were grown until confluence. Culture media consisted of stem cell media (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 1 × 10−6 M hydrocortisone succinate (Sigma, St Louis, MO), which was replaced weekly. After about 3 weeks of culture, stromal cells were trypsinized, washed, and placed in 48-well plates. After re-establishment of a confluent cellular layer, the plates were irradiated (15 Cy 250-kV x-rays) and used for further experiments. Cells were plated on preformed and irradiated stromal layers at varying cell densities. Freshly isolated CD34+ cells or total BMMCs were cocultured with the stromal layers for 5 weeks at 33°C. Media changes were performed weekly. After 5 weeks, the adherent cells were harvested by treatment with trypsin (Life Technologies, Gaithersburg, MD), washed, and replated in duplicate in methylcellulose with growth factor (GF) cocktail in order to estimate the numbers of cells able to form secondary colonies.
Cocultivation with agonistic and antagonistic Fas antibodies
After washing in Hanks balanced salt solution (HBSS; Life Technologies), BMMCs were resuspended in Iscove modified Dulbecco medium (IMDM; Life Technologies) and supplemented with 20% fetal calf serum (FCS; Life Technologies). The cells (0.2-1 × 106/mL) were then cultured in 20% IMDM containing a hematopoietic GF cocktail mix of 100 ng/mL interleukin-3 (IL-3; Amgen, Thousand Oaks, CA); 50 ng/mL granulocyte-macrophage colony-stimulating factor (Amgen); 50 ng/mL stem cell factor (SCF; Amgen); 5 U/mL erythropoietin (EPO); 50 ng/mL granulocyte colony-stimulating factor (Amgen); 50 ng/mL FLT-3 (Pepro Tech, Rocky Hill, NJ); and 10 ng/mL thrombopoietin (Pepro), with replacement of GF media on a weekly basis. Anti-Fas monoclonal antibody (mAb) CH11 (Immunotech, Marseilles, France), an antibody that mimics Fas-L by triggering the Fas receptor or the Fas-blocking antibody ZB4 (Immunotech), was used at 1 μg/mL where appropriate. FISH was performed on the specimens on days 0, 7, and 14 as previously described.
Results
Patients
Twenty-five patients with MDS were studied: 9 with monosomy 7, 14 with trisomy 8, and 2 with 5q− (Table1). In 7 cases, MDS had evolved from aplastic anemia: all of these patients had earlier been successfully treated with immunosuppressive drugs, and all were receiving cyclosporine at the time of study. No patient had radiation- or chemotherapy-related MDS.
Markers of apoptosis are up-regulated on total BM cells and on CD34 cells in trisomy 8
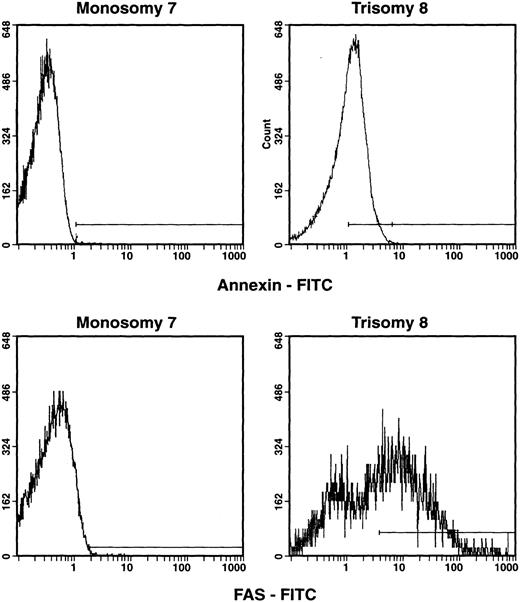
We first examined markers of Fas-mediated apoptosis in total BM and CD34 cells from patients with monosomy 7 and trisomy 8. T-cell–depleted BM cells from patients with trisomy 8 expressed significantly more cell-surface Fas than did those of patients with monosomy 7 (Table 2). In addition, the proportion of annexin+, propidium iodide–negative (PI−) cells was also increased in patients with trisomy 8 compared with normal controls or patients with monosomy 7. The number of apoptotic cells in many cases exceeded the number of trisomy 8 cells (Table 2). In a subgroup of 4 patients with trisomy 8 whose cells were stained for activated caspase-3, all showed significant increases when compared with healthy controls (Figures 1 and2). Numbers of caspase-3+cells were comparable to numbers of annexin+ cells determined by flow cytometry. To ensure that these findings were not related to differences in cellular differentiation (mature cells are more likely to express Fas), CD34 cells were isolated by sorting from these patients' marrow; selected CD34 cells from trisomy 8 patients also expressed increased amounts of Fas and annexin (n = 5; Figure 3). The CD34 cells as well as more primitive long-term colony-forming cells in all patients tested demonstrated cytogenetic abnormalities (Table3).
Expression of active caspase in cells with trisomy 8.
Freshly obtained BM smears from patients with trisomy 8 were stained for activated caspase as described in “Patients, materials, and methods,” then subsequently subjected to FISH for chromosome 8. Cells were scored for caspase and karyotype. The histograms show increased caspase staining in cells positive for trisomy 8 when compared with cells with a normal karyotype from the same patient. Normal BM demonstrated less than 3% caspase staining.
Expression of active caspase in cells with trisomy 8.
Freshly obtained BM smears from patients with trisomy 8 were stained for activated caspase as described in “Patients, materials, and methods,” then subsequently subjected to FISH for chromosome 8. Cells were scored for caspase and karyotype. The histograms show increased caspase staining in cells positive for trisomy 8 when compared with cells with a normal karyotype from the same patient. Normal BM demonstrated less than 3% caspase staining.
Example of a blood smear of a patient with trisomy 8 stained for activated caspase-3.
Freshly obtained blood smear was stained for activated caspase as described in “Patients, materials, and methods” and then subjected to FISH. A low-power view of cells staining for activated caspase is seen in panel A. A close-up view of a trisomy 8 cell, also positive for caspase-3, is seen in panel B. The same cell visualized with either a green or red bandpass filter showing caspase staining (C) and trisomy 8 probe (D). Original magnification × 100.
Example of a blood smear of a patient with trisomy 8 stained for activated caspase-3.
Freshly obtained blood smear was stained for activated caspase as described in “Patients, materials, and methods” and then subjected to FISH. A low-power view of cells staining for activated caspase is seen in panel A. A close-up view of a trisomy 8 cell, also positive for caspase-3, is seen in panel B. The same cell visualized with either a green or red bandpass filter showing caspase staining (C) and trisomy 8 probe (D). Original magnification × 100.
Apoptotic markers on CD34 cells obtained from patients with trisomy 8 and monosomy 7.
BMMNCs were obtained from patients with de novo MDS and trisomy 8 or monosomy 7 and were then stained with monoclonal antibodies: CD34-PE and annexin–fluorescein isothiocyanate (annexin-FITC) or CD95-FITC. Cells were gated for CD34 cells, and the number of annexin-staining and CD95-staining cells were quantitated. CD34+, CD95+, and CD34+, PI−, annexin+ cells are shown in the histograms above. CD34 cells from patients with trisomy 8 showed increased expression of annexin and CD95 when compared with patients with monosomy 7 (n = 5) and normal cells.10
Apoptotic markers on CD34 cells obtained from patients with trisomy 8 and monosomy 7.
BMMNCs were obtained from patients with de novo MDS and trisomy 8 or monosomy 7 and were then stained with monoclonal antibodies: CD34-PE and annexin–fluorescein isothiocyanate (annexin-FITC) or CD95-FITC. Cells were gated for CD34 cells, and the number of annexin-staining and CD95-staining cells were quantitated. CD34+, CD95+, and CD34+, PI−, annexin+ cells are shown in the histograms above. CD34 cells from patients with trisomy 8 showed increased expression of annexin and CD95 when compared with patients with monosomy 7 (n = 5) and normal cells.10
Expression of Fas-R is increased on cells with trisomy 8 but decreased in monosomy 7
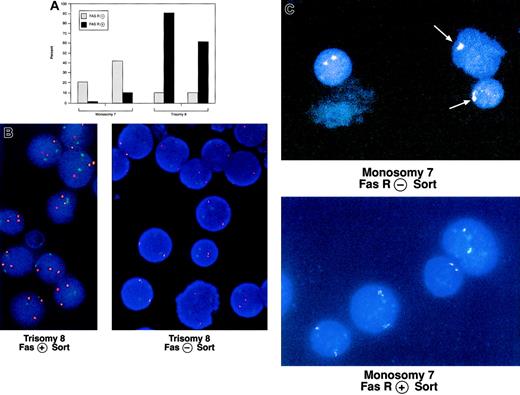
When CD3−, Fas+, and Fas− cells were separated and in situ hybridization was performed on the sorted cells by means of fluorescent probes specific for chromosomes 7 and 8, a disproportionate number of cells with trisomy 8 were found in the Fas+ population, while the number of cells with monosomy 7 were decreased in the Fas+ fraction (Figure4).
Comparison of CD95 expression on cells with trisomy 8 and monosomy 7 and normal cells from the same donor.
BM was obtained from 2 patients with trisomy 8 and 2 patients with monosomy 7 and stained with CD3-PE and CD95-FITC mAbs. CD3− cells were positively selected and sorted into CD95− and CD95+ aliquots, which were prepared for FISH with fluorescent probes for chromosome 7 or 8. The proportion of cells with each cytogenetic abnormality is shown in panel A. An example of stained cells in the Fas− and positive aliquots are seen in panel B (trisomy 8; chromosome 8 is orange; chromosome 7, a control, is green) and panel C (monosomy 7; chromosome 7). Trisomy 8 sample on the left was obtained from a patient with a history of aplastic anemia.
Comparison of CD95 expression on cells with trisomy 8 and monosomy 7 and normal cells from the same donor.
BM was obtained from 2 patients with trisomy 8 and 2 patients with monosomy 7 and stained with CD3-PE and CD95-FITC mAbs. CD3− cells were positively selected and sorted into CD95− and CD95+ aliquots, which were prepared for FISH with fluorescent probes for chromosome 7 or 8. The proportion of cells with each cytogenetic abnormality is shown in panel A. An example of stained cells in the Fas− and positive aliquots are seen in panel B (trisomy 8; chromosome 8 is orange; chromosome 7, a control, is green) and panel C (monosomy 7; chromosome 7). Trisomy 8 sample on the left was obtained from a patient with a history of aplastic anemia.
Effect of Fas agonist and antagonist on cytogenetically abnormal cells in tissue culture
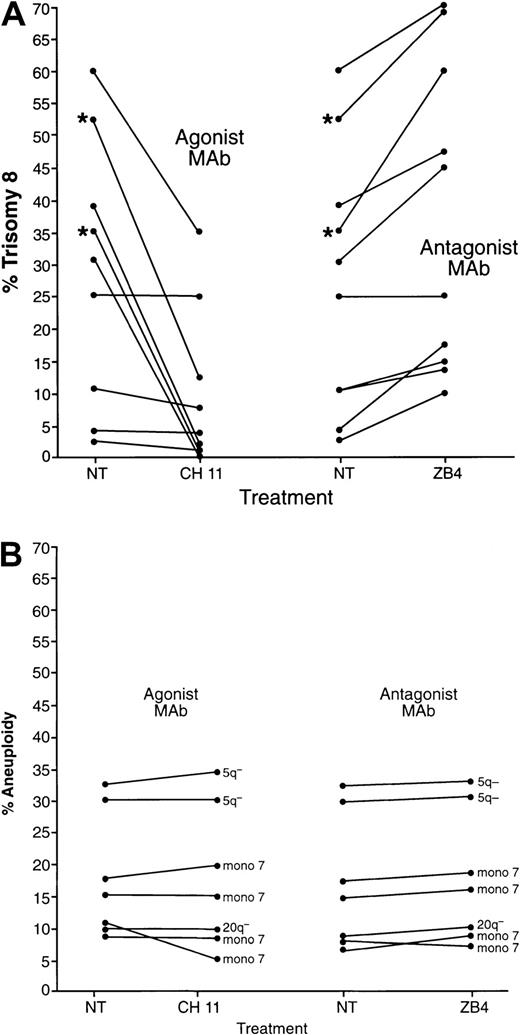
To assess the functional importance of high levels of Fas expression on trisomy 8 marrow cells, we determined if these cells were abnormally sensitive to the effects of Fas agonist. CH11, a monoclonal antibody that acts as a Fas agonist, and 2 mAbs ZB4 and M3, known to be Fas blocking antibodies, were added to BMMNCs of 9 MDS patients with trisomy 8, 4 patients with monosomy 7, and 2 patients with 5q−. Cells were cultured for 4 days in the presence of only GFs; of GFs and CH11; or of GFs and either ZB4 or M3. Seven of 9 samples from patients with trisomy 8 demonstrated decreases in the proportion of cells expressing trisomy 8 after Fas cross-linking (P < .05; Figure 5), while significant increases were observed when these cells were cultured with Fas antagonists, indicating a growth advantage for cells with trisomy 8 in the absence of Fas cross-linking (P < .01). T-cell depletion did not change the effect of Fas agonist on cultures of cells with trisomy 8 (data not shown; n = 4), indicating that Fas was present on the surface of bone marrow cells prior to culture. However, T-cell–depleted cells in short-term culture (not treated with Fas agonist or antagonist) all showed increases in trisomy 8 with time (n = 4), although most non–T-cell–depleted samples showed a decrease in the proportion of trisomy 8 with time (Tables4 and5). The 2 patients receiving CsA were the exception, showing increases in the percentage of trisomy 8 cells regardless of T-cell depletion. When BM cells from patients with monosomy 7 were studied in parallel experiments, no changes were seen in the percentage of cytogenetically abnormal cells (n = 4 for monosomy 7; n = 2 for 5q−; Figure 5B). Furthermore, when whole BM cells (not T-cell depleted) were cultured in media containing growth factors, there was a consistent decline in the number of cells expressing trisomy 8, while the percentage of cells with other cytogenetic abnormalities remained relatively constant (data not shown).
Proliferation of cells from patients with trisomy 8 and monosomy 7 in the presence of Fas agonist or Fas antagonist.
BMMNCs were cultured with and without Fas agonist monoclonal antibody mAb (CH11) or Fas antagonist mAb (ZB4). Slides were prepared, and FISH was performed with labeled centromeric probes for chromosomes 8 and 7 prior to culture and on days 4, 7, and 14. Days 0 and 4 are shown above (no change in expression was seen between days 4 and 14). The proportion of cells with trisomy 8 is seen in panel A; those with 5q− and monosomy 7 are seen in panel B. *Samples from patients with a history of aplastic anemia receiving CsA.
Proliferation of cells from patients with trisomy 8 and monosomy 7 in the presence of Fas agonist or Fas antagonist.
BMMNCs were cultured with and without Fas agonist monoclonal antibody mAb (CH11) or Fas antagonist mAb (ZB4). Slides were prepared, and FISH was performed with labeled centromeric probes for chromosomes 8 and 7 prior to culture and on days 4, 7, and 14. Days 0 and 4 are shown above (no change in expression was seen between days 4 and 14). The proportion of cells with trisomy 8 is seen in panel A; those with 5q− and monosomy 7 are seen in panel B. *Samples from patients with a history of aplastic anemia receiving CsA.
Discussion
About half of patients with primary MDS have cytogenetic abnormalities, the most common of which are trisomy 8, monosomy 7, and 5q−.11,12 Chromosomal changes may be observed at the onset of symptoms and/or evolve over time as disease progresses. Most patients develop MDS de novo without any pre-existing hematologic abnormality; in secondary MDS, cytotoxic chemotherapy or radiation exposure is a major factor in the induction of marrow failure and subsequent malignant transformation.13 MDS can also evolve from aplastic anemia.14,15 Specific cytogenetic abnormalities strongly correlate with prognosis in MDS. In general, patients with multiple cytogenetic abnormalities do poorly, and abnormalities involving chromosome 7 are especially hazardous.16,17 Patients with trisomy 8 have an intermediate prognosis, while patients with 5q− and 20q− have a better prognosis.18-20 In MDS following AA, trisomy 8 appears to confer a favorable prognosis.21
Because of the heterogeneity of the disease and the lack of clear markers for dysplasia, we examined BMs of MDS patients with specific cytogenetic abnormalities2 and showed that the factors affecting apoptosis and cell death of bone marrow cells appear to differ greatly for monosomy 7 and trisomy 8. BM CD34 cells with trisomy 8 showed more apoptosis than did CD34 cells with monosomy 7 or normal cells; Fas was much more likely to be highly expressed on cells with trisomy 8, while monosomy 7 cells were less likely to exhibit surface Fas than were normal cells or cells with trisomy 8. Although trisomy 8 cells were more sensitive to apoptosis than cells with normal karyotype, cells with normal karyotype obtained from the same patient still expressed greater than expected numbers of apoptotic markers in some cases. Activated caspase-3 was also found in cells with normal karyotype (though to a lesser extent than in trisomy 8 cells). These findings are consistent with an immune response directed against a neoantigen on trisomy 8 cells. In this scenario, activated T cells in proximity to trisomy 8 cells would release cytokines (interferon-γ and tumor necrosis factor–α), up-regulating Fas expression on the surface of hematopoietic cells. Nearby normal cells would be affected as innocent bystanders but to a lesser extent. A nonimmune mechanism in which the trisomy 8 cell is intrinsically more sensitive to apoptosis because of genetic programming cannot be rigorously excluded, although the increased frequency of apoptotic normal cells makes this hypothesis less likely. A third possibility is that the trisomy 8 clone may have arisen as part of a bone marrow failure state characterized by an apoptotic phenotype. Trisomy 8 cells may have subsequently gained a proliferative advantage. The fact that trisomy 8 cells have a proliferative advantage in the absence of an immune response is seen in T-cell–depleted samples, samples with Fas antagonist, and samples from patients receiving CsA therapy. A more thorough examination of these phenomena is under way.
While none of the trisomy 8 patients described here have progressed from marrow failure to malignant hematologic disease, 2 of the monosomy 7 patients developed acute myelogenous leukemia during a year of follow-up.21 Leukemic transformation has been presumed to be the result of multiple tandem chromosomal lesions and genetic mutations, some affecting cellular proliferation and others conferring resistance to apoptosis. Should a mutation that conferred resistance to apoptosis occur, the trisomy 8 clone would transform and expand. Cells with monosomy 7 exhibited resistance to apoptosis, and genetic alterations that affect proliferation would be favorable for these cells. Whether an increase in the size of the abnormal clone necessarily produces leukemia is unclear; 2 of our patients had trisomy 8 in at least 50% of their bone marrow cells, but they were asymptomatic except for requiring transfusion; their bone marrow continued to demonstrate normal maturation of their cells with very few blasts. Stability is not a feature of monosomy 7, as patients often progress to leukemia and die.21 While it would be expected that trisomy 8 would respond better to immunotherapy than patients in whom Fas-mediated apoptosis plays a smaller role, such therapy might lead to preferential expansion of cells with the cytogenetic abnormality. The long-term course of these patients is currently the subject of study.
Prepublished online as Blood First Edition Paper, August 15, 2002; DOI 10.1182/blood-2002-01-0096.
S.K. and M.F. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elaine M. Sloand, Bldg 10, Rm 7C103, National Institutes of Health, Bethesda, MD 20892-1652; e-mail:sloande@nhlbi.nih.gov.