CD45 is a membrane-associated tyrosine phosphatase that dephosphorylates Src family kinases and Janus kinases (JAKs). To clarify the role of CD45 in hematopoietic differentiation, we examined the effects of anti-CD45 monoclonal antibody NU-LPAN on the proliferation and differentiation of umbilical cord blood CD34+ cells. NU-LPAN showed a prominent inhibition of the proliferation of CD34+ cells induced by the mouse bone marrow stromal cell line MS-5 or erythropoietin (EPO). However, NU-LPAN did not affect the proliferation induced by interleukin 3. NU-LPAN also inhibited MS-5–induced or EPO-induced erythroid differentiation of CD34+ cells. The cells stimulated with EPO in the presence of NU-LPANmorphologically showed differentiation arrest at the stage of basophilic erythroblasts after 11 days of culture, whereas the cells treated with EPO without NU-LPAN differentiated into mature red blood cells. The Src family kinase Lyn and JAK2 were phosphorylated when erythroblasts obtained after 4 days of culture of CD34+ cells in the presence of EPO were restimulated with EPO. Overnight NU-LPAN treatment before addition of EPO reduced the phosphorylation of Lyn but not that of JAK2. Simultaneously, the enhancement of Lyn kinase activity after restimulation with EPO was reduced by NU-LPAN treatment. These results indicate selective inactivation of Lyn by CD45 activated with NU-LPAN and could partly explain the inhibitory mechanism on erythropoiesis exhibited by EPO. These findings suggest that CD45 may play a pivotal role in erythropoiesis.
Introduction
CD45 is a membrane-bound tyrosine phosphatase that dephosphorylates Src family kinases (Fyn, Lck, Lyn, Hck, etc)1-3 and plays a crucial role in T- and B-cell activation.4,5 CD45 dephosphorylates Fyn and Lck in mature T cells and Lyn in mature B cells at the COOH-terminal negative regulatory sites and then enhances the activities of these kinases.2,6,7 Interestingly, CD45 dephosphorylates the tyrosine residues of Lyn not only at the COOH-terminal negative regulatory sites but also at the positive regulatory site in the kinase domain in the case of the immature B-cell line WEHI-231.8,9 Lyn is activated by phosphorylation of the latter site, and dephosphorylation of both sites causes loss of Lyn kinase activity. Such negative regulation of Src family kinases is also observed in thymocytes.10 CD45 is also known to be involved in IgE-mediated degranulation of mast cells and integrin-mediated adhesion of macrophages.11 12 CD45 down-regulates the kinase activities of Lyn and Hck during adhesion of macrophages.
Irie-Sasaki et al13 14 recently reported a distinct function of CD45 as a phosphatase on Janus kinases (JAKs). Enhanced interleukin 3 (IL-3)–induced proliferation, and activation of JAKs and signal transducer and activators of transcription (STAT) proteins were observed in IL-3–dependent bone marrow–derived mast cell lines from CD45 gene-disrupted mice. In addition, CD45 directly dephosphorylated JAKs in vitro. Suppressive effects of CD45 signaling on erythropoietin (EPO)–mediated erythropoiesis and antiviral responses through inactivation of JAKs were also confirmed by a series of experiments using CD45 gene-disrupted mice and CD45-deficient Jurkat cells.
CD45 substrates, Src family kinases, and JAKs are also known to play important roles in cytokine receptor signaling in hematopoiesis.15,16 In particular, Lyn and JAK2 play pivotal roles in EPO-induced erythroid differentiation as signal transducers of the EPO receptor system.17-20 In this context, little is known about the role of CD45 in erythroid differentiation.2 14 We demonstrate here that the anti-CD45 monoclonal antibody NU-LPAN clearly hampered EPO-induced erythropoiesis but not IL-3–mediated cellular proliferation using umbilical cord blood (UCB) CD34+ cells.
Materials and methods
Cells
The UCB samples were obtained from clamped umbilical cords at full-term normal pregnancies. Approval was obtained from the institutional review board at the Kurashiki Medical Center for these studies. Informed consent was provided according to the Declaration of Helsinki. Mononuclear cells were isolated by density gradient centrifugation using Ficoll-Paque (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Selection of CD34+ cells was achieved with a magnetic activated cell sorting (MACS) CD34+ isolation kit and a mini-MACS column (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, Ficoll-Paque–separated cells were first labeled with hapten-conjugated anti-CD34 primary antibody (QBEND/10) and then mixed with magnetic beads conjugated to antihapten secondary antibody. These magnetically labeled cells were separated into CD34+and CD34− populations on a mini-MACS column. The column separation steps were repeated twice to obtain a highly enriched CD34+ population. Purity of the CD34+ cells was confirmed by flow cytometry using fluorescein isothiocyanate (FITC)–conjugated anti-CD34 antibody (8G12; BD Biosciences, San Jose, CA). Populations with higher than 90% purity of CD34+ cells were used for our experiments. Mouse bone marrow stromal cell line MS-521 was a generous gift from Dr K. J. Mori (Niigata University, Niigata, Japan) and was maintained in RPMI 1640 medium (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, Tokyo, Japan), 100 U/mL penicillin, and 50 μg/mL streptomycin (Sigma, St Louis, MO).
Coculture of CD34+ cells with MS-5 cells
MS-5 cells were plated at 1 × 104 cells/mL in 24-well tissue culture plates (Corning, Corning, NY) 1 week before coculture was started. CD34+ cells were resuspended into RPMI 1640 medium supplemented with 20% BIT9500 serum substitute (Stem Cell Technologies, Vancouver, BC, Canada) and antibiotics (100 U/mL penicillin and 50 μg/mL streptomycin) at a density of 1 × 104 cells/mL, with or without the addition of 20 μg/mL anti-CD45 monoclonal antibody NU-LPAN (Nichirei, Tokyo, Japan).22 The hybridoma culture supernatant was used as antibody source. The culture supernatant of MS-5 cells was replaced with CD34+ cell suspensions, and then the cells were cultured at 37°C in a humidified 5% CO2 atmosphere for the subsequent 2 weeks. Half of the medium was replaced with fresh medium on day 7. After 2 weeks, nonadherent cells were counted and harvested. Adherent cells were also harvested at the same time by detachment with trypsin-EDTA (ethylendiaminetetraacetic acid; Invitrogen) and mixed with nonadherent cells. Wright staining was performed on cytospin smears for morphologic examination and the remaining cells were tested for colony formation. To confirm the specificity of the NU-LPAN culture supernatant, protein G-Sepharose (Amersham Pharmacia Biotech)–purified NU-LPANand MOPC-21 immunoglobulin class–matched antibody (ICN Pharmaceuticals, Costa Mesa, CA) were used. Purified NU-LPAN was capable of reproducing the effect of the NU-LPAN culture supernatant, and MOPC-21 showed no effect on the proliferation and differentiation of CD34+ cells (data not shown).
Cytokine stimulation
CD34+ cells were resuspended into RPMI 1640 medium supplemented with 20% BIT9500 and antibiotics. The cells were plated at a density of 1 × 104 cells/mL for proliferation and colony formation assays, and at 1 × 105 cells/mL for flow cytometric analysis and determination of hemoglobin concentration with or without the addition of NU-LPAN. Stem cell factor (SCF), IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF; R & D Systems, Minneapolis, MN) were added to the cultures at a concentration of 100 ng/mL. EPO (Hayashibara Biochem Labs, Okayama, Japan) or thrombopoietin (TPO; R & D Systems) was added at concentrations of 5 U/mL and 50 ng/mL, respectively. After 5 to 11 days of culture, the number of cells was counted and cytospin smears were made for Wright staining.
Colony formation assay
Untreated CD34+ cells, cells after coculture with MS-5 or cells exposed to EPO were resuspended in Iscove modified Dulbecco medium (Invitrogen). Cell suspensions containing 500 to 2.5 × 104 cells in 100 μL volumes of medium were mixed with 1 mL cytokine-containing methylcellulose medium (MethoCult GF+ H4435; Stem Cell Technologies). After 2 weeks of culture at 37°C in a humidified 5% CO2 atmosphere, colonies were counted and classified into granulocyte-erythrocyte-macrophage-megakaryocyte colony-forming units (CFU-GEMMs), granulocyte-macrophage colony-forming units (CFU-GMs), or erythrocyte burst-forming units (BFU-Es) under the inverted microscope.
Flow cytometry
Expression of CD34 and glycophorin A on EPO-stimulated cells was determined by flow cytometry on an EPICS XL (Beckman Coulter, Fullerton, CA) using FITC-conjugated anti-CD34 antibody 8G12 and phycoerythrin-conjugated anti–glycophorin A antibody (GA-R2, BD Pharmingen, San Diego, CA), respectively.
Determination of hemoglobin concentration
Hemoglobin concentrations of EPO-stimulated cells were determined by colorimetric assay with diaminofluorene as described elsewhere.23
Preparation of cells for Western blotting, immunoprecipitation, and in vitro kinase assay
CD34+ cells stimulated with 5 U/mL EPO for 4 days were cultured in RPMI 1640 medium supplemented with 20% BIT9500 for 18 hours with or without the addition of 20 μg/mL NU-LPAN. The number of cells was adjusted to 0.8 to 2.5 × 106cells/mL and 500 to 1000 μL aliquots of the cell suspensions were seeded into reaction tubes in triplicate. After 15 minutes of incubation at 37°C, cells were stimulated with EPO (final concentration of 5 U/mL) for 0, 5, or 15 minutes.
Western blotting
The EPO-stimulated cells were harvested and washed once with ice-cold washing buffer solution containing 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl (pH 7.4), 2 mM sodium orthovanadate, 100 mM sodium fluoride, and 1 mM (P-amidinophenyl) methanesulfonyl fluoride hydrochloride. Cells were resuspended in 10 mL washing buffer solution and mixed with 10 μL sample buffer solution (4% sodium dodecyl sulfate [SDS], 20% glycerol, and 2 mM β-mercaptoethanol), and then heated at 100°C for 5 minutes for denaturation. Denatured samples were loaded onto 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes (Nitropure; Osmonics, Westborough, MA). The polyclonal antibodies rabbit anti-JAK2 (Upstate Biotechnology, Lake Placid, NY), rabbit antiphosphorylated JAK2 (Biosource International, Camarillo, CA), and rabbit anti-Lyn (Santa Cruz Biotechnology, Santa Cruz, CA) were used for the detection of JAK2, phosphorylated JAK2, and Lyn, respectively. A monoclonal mouse antiphosphorylated tyrosine antibody (4G10; Upstate Biotechnology) was used for the detection of phosphorylated Lyn. Polyclonal antibodies reactive against the respective primary antibodies conjugated to horseradish peroxidase (Dako, Glostrup, Denmark) were used as secondary reagents.
Immunoprecipitation and in vitro kinase assay
EPO-stimulated cells were harvested and washed once with ice-cold washing buffer solution. Pellets were lysed with 300 μL of a lysis solution containing 50 mM Tris-HCl (pH 7.4), 1% Triton X-100, 2 mM sodium orthovanadate, 100 mM sodium fluoride, and 1 mM (P-amidinophenyl) methanesulfonyl fluoride hydrochloride for 1 hour on ice. Lysates were centrifuged at 15 000g for 5 minutes at 4°C to remove nuclei and cell debris. Supernatants were incubated with a polyclonal rabbit anti-Lyn antibody for 3 hours at 4°C, and conjugates of antibody and Lyn proteins were coupled to protein G-Sepharose beads (Amersham Pharmacia Biotech) by incubation for 16 hours at 4°C. Immunoprecipitated beads were then harvested by centrifugation at 3500g for 1 minute at 4°C.
For the kinase reaction, immunoprecipitated beads were washed twice with ice-cold washing buffer solution, 3 times with kinase assay solution containing 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.5, 0.1 mM EDTA, and 0.015% Brij35, and then resuspended in 10 μL kinase assay buffer containing 0.1 mg/mL bovine serum albumin (BSA) and 0.2% β-mercaptoethanol. The beads suspension was mixed with 10 μL kinase assay buffer containing 2.5 μg Raytide EL protein kinase substrate or Raytide negative control substrate (Oncogene Research Products, Cambridge, MA). The kinase reaction was initiated by the addition of 10 μL of an adenosine triphosphate (ATP) mixture containing 0.15 mM ATP, 30 mM MgCl2, and 200 μCi/mL (7.4 MBq/mL) [γ-32P] ATP in kinase assay buffer. After incubation at 30°C for 30 minutes, the reaction was stopped by the addition of 120 μL 10% phosphoric acid. Immunoprecipitated beads were removed by centrifugation at 15 000 rpm for 1 minute, and 120 μL supernatant was applied onto a square (2 × 2 cm) of P81 paper. The paper was extensively washed with 0.5% phosphoric acid, and then relative quantity of [32P] incorporated into phosphorylated protein kinase substrates was measured by liquid scintillation counter (LSC-740; Aloka, Tokyo, Japan).
For quantification of Lyn, part of the immunoprecipitated beads was resuspended in 10 μL sample buffer solution and heated at 100°C for 5 minutes for denaturation. Denatured samples were loaded onto 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. The monoclonal antibody mouse anti-Lyn (Santa Cruz Biotechnology) was used for detection of Lyn. Polyclonal antibody reactive against the primary antibody conjugated to horseradish peroxidase was used as a secondary reagent.
Results
Inhibition of MS-5–induced proliferation and differentiation by NU-LPAN
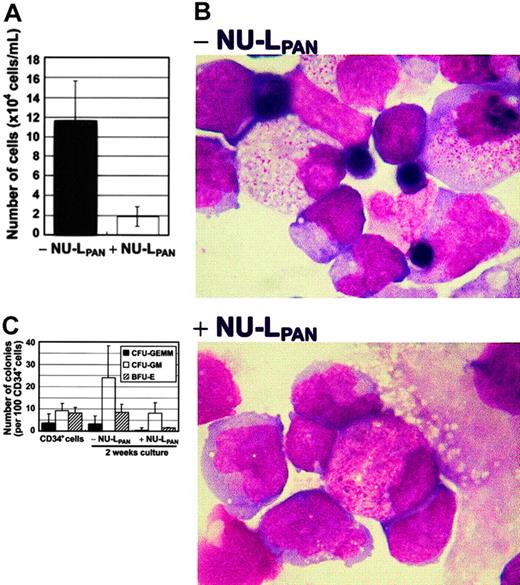
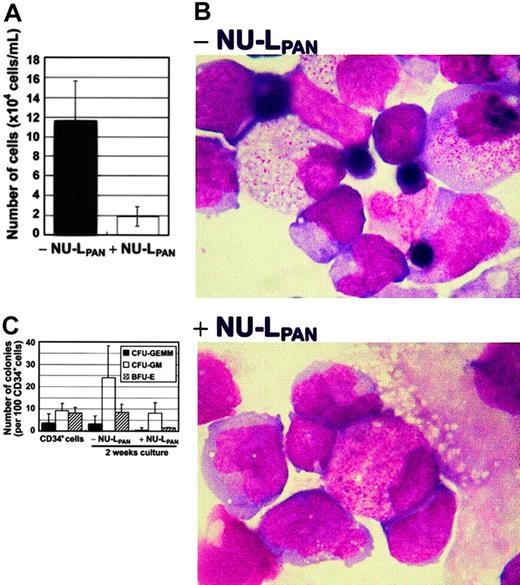
The ability of the mouse bone marrow stroma cell line MS-5 to induce growth and differentiation of human hematopoietic cells has been reported elsewhere.21 MS-5 supported UCB CD34+cell proliferation 11.9-fold during 2 weeks of culture. Culture gave rise to mixed lineages of myeloid and erythroid cell populations. The stimulation of CD34+ cells with NU-LPAN reduced the proliferation to 1.92-fold (Figure1A). NU-LPAN also inhibited MS-5–induced erythroid differentiation of CD34+ cells (Figure 1B). Moreover, CFU-GEMMs and BFU-Es were almost completely absent in the presence of NU-LPAN (Figure 1C).
MS-5–induced proliferation and differentiation of CD34+ cells.
CD34+ cells were plated at 1 × 104 cells/mL on MS-5 feeder cells with or without the addition of 20 μg/mL NU-LPAN. After 2 weeks, the number of cells was determined (A). NU-LPAN prohibited erythroid maturation as demonstrated by Wright staining (B); original magnification, × 125. CD34+ cells and cultured cells were subjected to colony formation assay and the numbers of each CFU-GEMM, CFU-GM, and BFU-E were determined (C). For panels A and C, data are represented as the means ± SD (n = 3).
MS-5–induced proliferation and differentiation of CD34+ cells.
CD34+ cells were plated at 1 × 104 cells/mL on MS-5 feeder cells with or without the addition of 20 μg/mL NU-LPAN. After 2 weeks, the number of cells was determined (A). NU-LPAN prohibited erythroid maturation as demonstrated by Wright staining (B); original magnification, × 125. CD34+ cells and cultured cells were subjected to colony formation assay and the numbers of each CFU-GEMM, CFU-GM, and BFU-E were determined (C). For panels A and C, data are represented as the means ± SD (n = 3).
Inhibition of EPO-induced proliferation and erythroid differentiation by NU-LPAN
SCF, IL-3, G-CSF, EPO, and TPO all independently induced the proliferation of CD34+ cells (Figure2). GM-CSF was also tested; however, no significant induction of CD34+ cell proliferation was observed (Figure 2C). NU-LPAN showed a clear inhibition of EPO-induced proliferation, and the number of cells was reduced from 1.23 × 106 to 5.20 × 104 cells/mL after 11 days of culture in the presence of NU-LPAN (Figure 2E). NU-LPAN slightly affected the proliferation of cells stimulated with SCF, G-CSF, and TPO (Figure 2A,D,F). However, NU-LPAN did not show any effect on IL-3–induced proliferation (Figure 2B). In addition, NU-LPAN clearly inhibited EPO-induced erythroid differentiation of CD34+cells (Figure 3). Although clear erythroid proliferation and differentiation were observed in the presence of EPO without NU-LPAN for 8 to 11 days, the majority of cells in culture in the presence of NU-LPANshowed immature erythroid morphology even after 11 days in culture (Figure 3). The cells stimulated with SCF, IL-3, GM-CSF, G-CSF, and TPO, with or without the addition of NU-LPAN, were morphologically similar (data not shown).
Cytokine-induced proliferation of CD34+cells.
CD34+ cells were plated at 1 × 104 cells/mL with the addition of 100 ng/mL SCF (A), 100 ng/mL IL-3 (B), 100 ng/mL G-CSF (C), 100 ng/mL GM-CSF (D), 5 U/mL EPO (E), or 50 ng/mL TPO (F). The number of cells was counted after 5 to 11 days in culture. ○ (NU-LPAN+) and ● (NU-LPAN−) indicate cells that were cultured with and without the addition of NU-LPAN, respectively. For all panels, data are represented as the means ± SD (n = 3).
Cytokine-induced proliferation of CD34+cells.
CD34+ cells were plated at 1 × 104 cells/mL with the addition of 100 ng/mL SCF (A), 100 ng/mL IL-3 (B), 100 ng/mL G-CSF (C), 100 ng/mL GM-CSF (D), 5 U/mL EPO (E), or 50 ng/mL TPO (F). The number of cells was counted after 5 to 11 days in culture. ○ (NU-LPAN+) and ● (NU-LPAN−) indicate cells that were cultured with and without the addition of NU-LPAN, respectively. For all panels, data are represented as the means ± SD (n = 3).
Morphology of EPO-stimulated CD34+ cells.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (B,D,F) or without (A,C,E) the addition of 20 μg/mL NU-LPAN. After 5 to 11 days in culture (as indicated), Wright staining was performed on cytospin smears. Original magnification for all panels, × 125.
Morphology of EPO-stimulated CD34+ cells.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (B,D,F) or without (A,C,E) the addition of 20 μg/mL NU-LPAN. After 5 to 11 days in culture (as indicated), Wright staining was performed on cytospin smears. Original magnification for all panels, × 125.
Characterization of NU-LPAN–stimulated cells
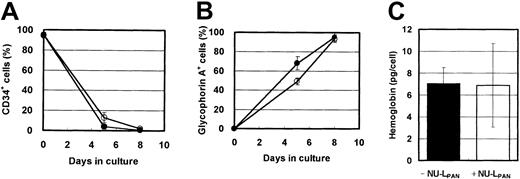
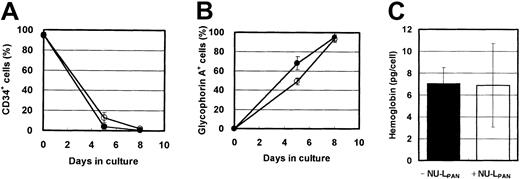
In the presence of EPO, the expression of CD34 and glycophorin A was down-regulated and up-regulated, respectively. NU-LPANslightly prolonged these effects (Figure4A-B). However, more than 90% of the cells were CD34−, glycophorin A+ after 8 days in cultures both with and without NU-LPAN, and hemoglobin synthesis in the cells cultured with or without the addition of NU-LPAN was similar (Figure 4). Based on the reports by Okumura et al24 and Nakahata et al,25morphologic and immunologic characteristics indicated that the NU-LPAN–stimulated cells were compatible with basophilic erythroblasts.
Phenotypic characteristics of EPO-stimulated cells.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (○ and ■) or without (● and ▪) the addition of 20 μg/mL NU-LPAN. After 5 or 8 days in culture, the expressions of CD34 (A) and glycophorin A (B) were analyzed. The concentration of hemoglobin in the cultured cells was measured after 8 days in culture (C). For all panels, data are represented as the means ± SD (n = 3).
Phenotypic characteristics of EPO-stimulated cells.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (○ and ■) or without (● and ▪) the addition of 20 μg/mL NU-LPAN. After 5 or 8 days in culture, the expressions of CD34 (A) and glycophorin A (B) were analyzed. The concentration of hemoglobin in the cultured cells was measured after 8 days in culture (C). For all panels, data are represented as the means ± SD (n = 3).
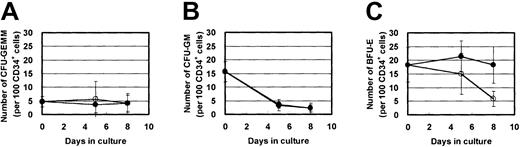
In addition to the above effect of NU-LPAN on erythroid differentiation, NU-LPAN also affected the proliferation of immature erythroid progenitor cells. The number of BFU-Es after EPO stimulation was decreased after addition of NU-LPAN; however, NU-LPAN did not affect CFU-GEMMs and CFU-GMs (Figure 5).
NU-LPAN specifically decreases the number of BFU-Es.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (○) or without (●) the addition of 20 μg/mL NU-LPAN. After 0, 5, or 8 days in culture, cells were subjected to the colony formation assay and the numbers of CFU-GEMMs (A), CFU-GMs (B), or BFU-Es (C) were determined. For all panels, data are represented as the means ± SD (n = 3).
NU-LPAN specifically decreases the number of BFU-Es.
CD34+ cells were plated at 1 × 104 cells/mL and cultured in medium containing 5 U/mL EPO, with (○) or without (●) the addition of 20 μg/mL NU-LPAN. After 0, 5, or 8 days in culture, cells were subjected to the colony formation assay and the numbers of CFU-GEMMs (A), CFU-GMs (B), or BFU-Es (C) were determined. For all panels, data are represented as the means ± SD (n = 3).
Inhibition of EPO-induced phosphorylation of Lyn by NU-LPAN
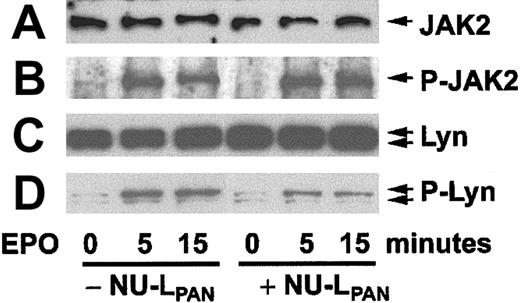
When erythroblasts obtained after 4 days culture of CD34+ cells in the presence of EPO were restimulated with EPO, the cells showed phosphorylation of JAK2 and Lyn (Figure6). Preincubation of the cells with NU-LPAN for 18 hours prior to EPO restimulation reduced the phosphorylation of Lyn but not that of JAK2 (Figure 6). Corresponding with the reduced level of phosphorylation, the kinase activity of Lyn was also inhibited by the NU-LPAN treatment (Figure7).
Phosphorylation of JAK2 and Lyn.
CD34+ cells stimulated with EPO for 4 days were again incubated with or without the addition of NU-LPAN for 18 hours. Incubated cells were restimulated with 5 U/mL EPO for 0, 5, and 15 minutes, and then cell lysates were prepared. JAK2 (A), phosphorylated JAK2 (B), Lyn (C), and phosphorylated Lyn (D) were detected by Western blotting using anti-Jak2, antiphosphorylated JAK2, anti-Lyn and antiphosphorylated tyrosine antibodies, respectively. For the detection of phosphorylated Lyn bands by antiphosphorylated tyrosine antibody, the bands corresponding to the molecular weight of Lyn were inferred to as phosphorylated Lyn. A result representative of 3 separate experiments is presented.
Phosphorylation of JAK2 and Lyn.
CD34+ cells stimulated with EPO for 4 days were again incubated with or without the addition of NU-LPAN for 18 hours. Incubated cells were restimulated with 5 U/mL EPO for 0, 5, and 15 minutes, and then cell lysates were prepared. JAK2 (A), phosphorylated JAK2 (B), Lyn (C), and phosphorylated Lyn (D) were detected by Western blotting using anti-Jak2, antiphosphorylated JAK2, anti-Lyn and antiphosphorylated tyrosine antibodies, respectively. For the detection of phosphorylated Lyn bands by antiphosphorylated tyrosine antibody, the bands corresponding to the molecular weight of Lyn were inferred to as phosphorylated Lyn. A result representative of 3 separate experiments is presented.
Kinase activity of Lyn.
CD34+ cells stimulated with EPO for 4 days were incubated with or without the addition of NU-LPAN for a further 18 hours. Incubated cells were stimulated with 5 U/mL EPO for 0, 5, and 15 minutes, after which Lyn proteins were immunoprecipitated. Lyn (A) was detected using anti-Lyn antibody. The relative activity of Lyn was calculated from the radioactivity count of [32P] incorporated into phosphorylated Raytide EL, the tyrosine kinase substrate (B). A result representative of 5 separate experiments is presented.
Kinase activity of Lyn.
CD34+ cells stimulated with EPO for 4 days were incubated with or without the addition of NU-LPAN for a further 18 hours. Incubated cells were stimulated with 5 U/mL EPO for 0, 5, and 15 minutes, after which Lyn proteins were immunoprecipitated. Lyn (A) was detected using anti-Lyn antibody. The relative activity of Lyn was calculated from the radioactivity count of [32P] incorporated into phosphorylated Raytide EL, the tyrosine kinase substrate (B). A result representative of 5 separate experiments is presented.
Discussion
CD45 is known as a membrane-bound tyrosine phosphatase that regulates T- and B-cell activation through dephosphorylation of Src family kinases.1-5 CD45 is expressed on most hematopoietic cells except for mature red blood cells and platelets. However, the role of CD45 in hematopoietic differentiation has only been partially addressed.1,2,14 Irie-Sasaki et al13 and Penninger et al14 reported on a novel function of CD45 as a phosphatase of JAKs. They reported that CD45 down-regulated the proliferation and differentiation of hematopoietic cells through dephosphorylation of JAKs. JAKs have been known to play an indispensable role in the signaling pathways of most hematopoietic cytokine receptors including those of EPO and IL-3 receptors.15 16
To confirm the role of CD45 in hematopoietic differentiation, we investigated the effects of the anti-CD45 antibody, NU-LPAN, using UCB CD34+ cells as a target. NU-LPAN was found to strongly inhibit the proliferation and differentiation of CD34+ cells (Figure 1). As a possible mechanism, we first considered dephosphorylation of JAKs induced by CD45 signaling. However, NU-LPAN clearly hampered EPO-induced proliferation and erythroid differentiation of CD34+ cells but not that of IL-3–induced proliferation of the cells (Figures 2-5). In fact, NU-LPAN did not inhibit EPO-induced phosphorylation of JAK2 (Figure 6). Our experiments showed that CD45 signaling inhibited erythropoiesis in a manner rather different from the mechanism of inhibition of JAKs.
On the other hand, preincubation of cells with NU-LPAN for 18 hours prior to EPO stimulation revealed a reduced level of phosphorylated Lyn (Figure 6). The reduced level of phosphorylation correlates with the reduced kinase activity of Lyn (Figure 7). CD45 is known as a phosphatase that acts on Src family kinases including Lyn, and either positively or negatively controls the said kinase activities through the dephosphorylation of tyrosine residues at positive or negative regulatory sites as described in the “Introduction.” Therefore, we concluded that CD45 is activated by the binding with NU-LPAN and continuously dephosphorylates the positive regulatory site on Lyn to inhibit its kinase activity. Lyn is known to associate with various cytokine receptors such as those for EPO and IL-3.17-20,26,27 Although important roles for Lyn in EPO-induced erythroid differentiation have been described, the role for Lyn in IL-3–induced proliferation and differentiation of hematopoietic cells is still unknown.16-20 Tilbrook et al18 reported that an immature erythroid cell line J2E has the ability to differentiate into mature erythrocytes after EPO stimulation. A mutant clone of J2E, J2E-NR, was originally isolated due to its inability to differentiate in response to EPO. J2E-NR showed markedly reduced expression of Lyn, and transfection of J2E-NR with a retroviral vector carrying Lyn restored its responsiveness to EPO. Dominant-negative forms of Lyn transfected into J2E showed erythroid differentiation arrest.19 Other researchers reported an association between Lyn and the EPO receptor and a role for Lyn in the STAT5 signaling pathway using an EPO receptor–expressing clone of 32D cells and the human erythroleukemia cell line F36P.20Those reports support the inhibition of erythroid differentiation by CD45 signaling through association with selective inactivation of Lyn.
Although Lyn must be a major CD45 substrate in inhibition of erythroid differentiation, there is still remaining uncertainty whether Lyn is the only substrate or not. Hibbs et al28 reported the establishment of a Lyn knockout mouse; however, the mouse had the ability to produce cells of all hematopoietic lineages. This experiment suggests that there are alternative erythroid differentiation-inducing pathways independent of Lyn involvement. NU-LPAN completely inhibited erythroid differentiation of CD34+ cells, suggesting that CD45 also inhibits such an alternative pathway.
The specific effect of NU-LPAN on the erythroid lineage should be due to the substrate specificity of CD45. However, the mechanism by which CD45 selectively dephosphorylates Lyn but not JAK2 has not yet been identified. Nevertheless, it is assumed that the epitope on CD45 recognized by NU-LPAN must be involved in the inhibitory mechanism.
CD45 is a glycosylated cell surface molecule consisting of 8 isoforms resulting from alternative splicing of 3 exons, A, B, and C.1,22,29 The primitive CD34+ cells in both the UCB and bone marrow are presumed to express CD45R0 and RB, but low to undetectable levels of RA.29 During erythroid differentiation, CD34+ cells remain CD45RO and RB positive, whereas they lose this expression of RB and will be positive for CD45RA after commitment to the myeloid or lymphoid lineages.29Because NU-LPAN is an anti-CD45 antibody recognizing all isoforms, several questions remain regarding whether NU-LPAN affected erythroid cells directly through CD45RB or not. However, NU-LPAN specifically affects cells of the erythroid lineage carrying CD45RB. This result suggests that the effect of NU-LPAN through CD45RB might be involved in the Lyn-specific inhibitory effect induced by CD45 signaling.
There are some reports stating that various types of CD45 antibodies induce intracellular signaling.30,31 UCHL-1, which is specific to CD45RO but not GAP8.3, which is specific to CD45, inhibited IL-2– or IL-4–induced proliferation of T cells.30 This could be due to different effects of the antibodies on STAT and extracellular signal-related kinase activity. In contrast, GAP8.3 but not UCHL-1 inhibited CD3-mediated proliferation of quiescent T cells.30 T29/33, which is an antibody specific to CD45 (kindly provided by Dr I. S. Trowbridge, The Salk Institute, La Jolla, CA), showed clear inhibition of EPO-induced proliferation of CD34+ cells similar to NU-LPAN (data not shown). This result shows that the effects observed in this study are due to CD45 itself and are not artifactual due to other nonspecific effects of NU-LPANantibody. However, Broxmeyer et al31 reported results discordant from our experiments showing that certain anti-CD45 antibodies inhibit the proliferation of myeloid progenitors but not that of erythroid progenitors. Thus, CD45 signaling varies between different target cells and antigenic epitopes. We show here that NU-LPAN inhibits erythroid differentiation of UCB CD34+ cells associated with selective inactivation of Lyn. However, there are still uncertainties remaining whether inactivation of Lyn is restricted to the combination of UCB CD34+ cells and NU-LPAN antibody. Investigation of CD45 signaling using other combinations of different cell types and antibodies is important for the total understanding of the mechanism by which CD45 controls the proliferation and differentiation of hematopoietic cells. Investigation of natural ligands of CD45 is of great interest. CD22 and galectin are reportedly candidates for the natural ligand of CD45 due to their binding capacity with the sugar residue of CD45; however, there remain several uncertainties in this hypothesis as well.14,32 33Our results might simulate other possible natural ligands that affect erythroid differentiation like NU-LPAN does.
Although there are still a number of remaining unknowns regarding erythroid differentiation, EPO acts as an excellent erythroid potentiator in vitro as well as in vivo. We have demonstrated here CD45 plays a pivotal role in the negative regulation of erythroid proliferation and differentiation in vitro. NU-LPAN could be a useful material for further investigation into the negative regulatory mechanisms in erythropoiesis.
We thank M. J. Micallef for a critical review of the manuscript.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-03-0864.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Akira Harashima, Fujisaki Cell Center, Hayashibara Biochemical Labs, 675-1 Fujisaki, Okayama 702-8006, Japan; e-mail: fcch@hayashibara.co.jp.



![Fig. 7. Kinase activity of Lyn. / CD34+ cells stimulated with EPO for 4 days were incubated with or without the addition of NU-LPAN for a further 18 hours. Incubated cells were stimulated with 5 U/mL EPO for 0, 5, and 15 minutes, after which Lyn proteins were immunoprecipitated. Lyn (A) was detected using anti-Lyn antibody. The relative activity of Lyn was calculated from the radioactivity count of [32P] incorporated into phosphorylated Raytide EL, the tyrosine kinase substrate (B). A result representative of 5 separate experiments is presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-03-0864/4/m_h82423513007.jpeg?Expires=1763553669&Signature=XTJiz1QAu7HR19ojounQ9oGPxOiDbgPqq55N1cR4lvK4V2BJPyGWCgo1wwSwXzLDjWeeBIuo2t-FSJaIE1Y503FNU0V7ulFdq~x4StI4qd2VfuAwV0YDibT3ORDmpPs3wW0nRvj~ZESaX35w1~dyJRNf~r7HGl-jM8gFv4zBjFO0m0A1i87xzFlc9~rQZ7TTw8I6DCM-S9B4Zyj73M91nX3zj-gkTr4h1hbJm-ayNh0HcZ4AelZCuj-4NK9odtZQNXeTsWtSip4R6ikWwXSmBxkAl2nwhAHUaDEfNL2WHiNWeUKY3xah6TjMS-OCQ0nV79MEgXDAJynRUB3FfkcPTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 7. Kinase activity of Lyn. / CD34+ cells stimulated with EPO for 4 days were incubated with or without the addition of NU-LPAN for a further 18 hours. Incubated cells were stimulated with 5 U/mL EPO for 0, 5, and 15 minutes, after which Lyn proteins were immunoprecipitated. Lyn (A) was detected using anti-Lyn antibody. The relative activity of Lyn was calculated from the radioactivity count of [32P] incorporated into phosphorylated Raytide EL, the tyrosine kinase substrate (B). A result representative of 5 separate experiments is presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/13/10.1182_blood-2002-03-0864/4/m_h82423513007.jpeg?Expires=1763553670&Signature=vrLfXDZhpDQwTTxj8ef2E-J08ZWstaBrpWKaExQN629law2e7W1UUmHtPHG5JBEw49Gz2Ly7dJYUAtYSSXXOHEgIrjhJUYYMCzAxsxDO0q4iCHWg4tcX3~orS0Ns2Pzrk75Ie-ZsV3FkPN4kahzZDaWl5o4Lvn1-w6iMCmw5Bo-l~Oc1sLJ-dGYUtRvIQgETAModHzW3jUvoSDv4YJzPLtiPn6erQPDJ7-wyQw0CD2LyT-XRJDudhnK0RAXOTqjWPM-yjgJ0Tcp-x5uK2T8IlA2Lba2RbwotFvXwBTOMtex53it0j25TGIHUIZYpaEWnb34musLmERd9FfHE9kXYTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)