Invasive aspergillosis has become a major cause of infection-related mortality in nonneutropenic patients after allogeneic stem cell transplantation (SCT). To assess the potential role ofAspergillus-specific T-cell responses for the successful control of invasive aspergillosis, lymphoproliferative responses toAspergillus fumigatus antigens were studied in healthy individuals, patients with evidence of invasive aspergillosis, and patients late after allogeneic SCT. In healthy individuals, a positive lymphoproliferative response was documented to cellular extracts of A fumigatus (14 of 16), the 88-kDa dipeptidylpeptidase (4 of 16), and the 90-kDa catalase (8 of 11). A predominant release of interferon γ (IFN-γ) in culture supernatants on stimulation with A fumigatus antigens was demonstrated in 13 of 17 healthy individuals, indicating a TH1 response. In patients with clinical evidence of invasive aspergillosis, a favorable response to antifungal therapy was found to correlate with a higher IFN-γ/interleukin 10 (IL-10) ratio in culture supernatants (n = 7; median ratio, IFN-γ/IL-10 = 1.0; range, 0.09-24.8) compared to 10 patients with progressive or stable disease (median ratio, IFN-γ/IL-10 = 0.1; range, 0.002-2.1; P = .04). Steroid treatment was found to suppressAspergillus-specific lymphoproliferation (P = .037) and release of IFN-γ in culture supernatants (P = .017). In contrast to cytomegalovirus- and tetanus toxoid–specific T-cell responses, Aspergillus-specific T-cell reconstitution late after allogeneic SCT was characterized by low stimulation indices and a low IFN-γ/IL-10 ratio. In addition, phosphoantigen-reactive Vγ9/Vδ2 T-cell clones from healthy individuals were found to produce significant amounts of tumor necrosis factor in response to A fumigatus antigens. In conclusion, these results further support the hypothesis that T cells contribute to the host defense against A fumigatus.
Introduction
Invasive aspergillosis has become a major cause of infection-related mortality in patients with hematologic malignancies, especially after allogeneic stem cell transplantation (SCT).1 In the immunocompromised patient, invasive aspergillosis most frequently affects the lungs and is characterized by hyphal invasion and destruction of pulmonary tissue. Proven risk factors in humans are defects in phagocyte function,2steroid-induced suppression of macrophage conidiocidal activity,3 and chemotherapy-induced neutropenia.4 More recently, invasive aspergillosis has been reported with an increasing frequency in nonneutropenic patients with advanced AIDS,5,6 in preterm neonates,7and in patients after solid organ transplantation and allogeneic SCT.1
Airborne transmission of fungal spores has been considered to be the major route of transmission of invasive aspergillosis in the immunosuppressed host. Patients with a previous history of invasive aspergillosis have been found to be at an increased risk for recurrence of invasive aspergillosis during a subsequent episode of neutropenia or immunosuppression.8 These data and results from our group demonstrating Aspergillus DNA in lower respiratory tract samples to be an important risk factor for the development of invasive aspergillosis during a subsequent episode of immunosuppression indicate that a subset of patients is obviously colonized without signs of tissue-invasive disease.9 These observations imply that local cellular defects in the innate and adaptive immune effector mechanisms are major predisposing factors of the host to invasive aspergillosis.3,10 11
In the murine model of invasive pulmonary aspergillosis, resistance to the infection was associated with production of tumor necrosis factor α (TNF-α), interleukin 12 (IL-12), and interferon γ (IFN-γ).10,12,13 Production of the TH2 cytokines IL-4 and IL-10 by interstitial lymphocytes was associated with disease progression.10,12 The development of TH1 protective immunity also correlated with resistance to subsequent lethal infection.12,14 More recently, the adoptive transfer of CD4+ splenocytes from mice sensitized to a crude culture filtrate of Aspergillus fumigatus into naive animals was found to significantly prolong survival after a subsequent intravenous challenge with A fumigatusconidia.15
In the study reported, we assessed the lymphoproliferative T-cell response to various A fumigatus antigens in healthy individuals, patients with evidence of invasive aspergillosis, and patients late after allogeneic SCT. In the vast majority of healthy individuals and in patients surviving invasive aspergillosis, a significant lymphoproliferative response to A fumigatusproteins was found with a dominant release of IFN-γ in culture supernatants. In patients late after allogeneic SCT,Aspergillus-specific T-cell reconstitution was characterized by low stimulation indices (SIs) and a low IFN-γ/IL-10 ratio. Thus, this is the first study reporting on the important role of a TH1-type cellular immune response for the control of invasive aspergillosis in patients with hematologic malignancies. In addition to T-helper cell responses, also phosphoantigen-reactive Vγ9/Vδ2 T-cell clones were found to produce significant amounts of TNF in response to A fumigatus antigens.
Patients, materials, and methods
Cell preparation
Peripheral blood mononuclear cells (PBMNCs) of healthy donors and hematologic patients with clinical evidence of invasive aspergillosis were separated by use of Ficoll-Hypaque density gradient centrifugation (Linaris, Bettingen am Main, Germany), washed twice in sterile calcium- and magnesium-free Hanks balanced salt solution (PAA Laboratories, Linz, Austria), and resuspended in RPMI 1640 medium supplemented with Glutamax-I, 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (Gibco BRL, Grand Island, NY), 200 μg/mL gentamicin sulfate (Refobacin, 80 mg, Merck, Darmstadt, Germany), and 10% fetal calf serum (FCS; Sigma, St Louis). All cell preparations were more than 95% viable as judged by trypan blue dye.
A fumigatus antigens
Conidia of A fumigatus (strain CBS 144-89) were inoculated in 150-mL Erlenmeyer flasks containing Sabouraud liquid medium (2% [wt/vol] glucose, 1% [wt/vol] mycopeptone). Flasks were shaken for 24 hours at 37°C and 200 rpm. Two-liter fermenters (LSL Biolafitte, Saint Germain en Laye, France) containing 1.2 L Sabouraud medium were inoculated with the shaken flask cultures. The 18-hour culture conditions were as follows: inoculum 8% (vol/vol); temperature, 26°C; aeration, 50 L air/min; agitation, 500 rpm. The mycelial mat recovered by filtration was extensively washed with water. Mycelium was disrupted in a glass bead cell homogenizer in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl buffer pH 7.5 and the water-soluble cellular extracts (EC SAB) were recovered after centrifugation. Protein content was measured by the Bio-Rad technique according to the manufacturer's instructions (Bio-Rad, Marne La Coquette, France) and estimated in milligram equivalent bovine serum albumin (BSA)/mL.
Conidia of A fumigatus were harvested after 3 days of culture on Sabouraud dextrose agar (Difco, Detroit, MI), filtered through sterile gauze, killed by heating in a water bath at 100°C for 1 hour, washed with saline solution, and stored at −4°C. Heat-killedA fumigatus conidia were tested for sterility by subculturing on Sabouraud dextrose agar for 10 days.
Lymphoproliferation assay
The proliferation assay was performed as described before.18 The A fumigatus antigens were added to the wells in concentrations ranging from 50 μg to 50 ng protein/mL. Conidia were tested in lymphoproliferative assays at concentrations ranging from 5 × 105 to 5 × 102. Tetanus toxoid (Chiron Behring, Marburg, Germany), cytomegalovirus (CMV) antigen (Biodesign, Dunn, Asbach, Germany), phytohemagglutinin (PHA; Murex, Life Technology, Karlsruhe, Germany), and IL-2 (Biotest, Dreieich, Germany) were used as control T-cell stimuli and added at final concentrations of 20 μg/mL, 500 ng/mL, 10 ng/mL, and 50 U/mL, respectively. T cells were stimulated with antigen for 5 days and 1 μCi (37 KBq)3H-thymidine was added overnight. An SI of 3 or higher was considered to indicate a positive lymphoproliferative response.
MACS
For magnetic-activated cell sorting (MACS) separation (Miltenyi Biotec, Bergisch Gladbach, Germany), isolated mononuclear cells (MNCs) from anticoagulated human blood were labeled with MACS CD4 or CD8 MicroBeads, incubated for 15 minutes at 8°C, and washed extensively. Thereafter, cell suspensions were passed through a positive selection column type MS+ that was placed in the magnetic field of a MiniMACS separator. After removal of the column from the magnetic field, the magnetically retained CD4+ or CD8+ cells could be eluted as a positively selected cell fraction. The unlabeled cell fraction was depleted of CD4+and CD8+ cells.
Cytokine determination
PBMNCs (105/200 μL), A fumigatusantigen EC SAB (5μg/mL), or the 90-kDa catalase (5 μg/mL) were cultured in 96-well, round-bottomed plates. After 5 days of culture, the supernatant was removed from each well and stored at −80°C. Supernatants were tested for IL-10 and IFN-γ by use of commercial antigen-capture enzyme-linked immunosorbent assay (ELISA) kits (IL-10 ELISA, DPC Biermann, Bad Nauheim, Germany; IFN-γ ELISA, Biozol Diagnostica, Echingen, Germany). In case of cytokine concentrations below the analytical sensitivity of the assay, a concentration of 1 pg/mL for the respective cytokines was used to calculate the IFN-γ/IL-10 ratio.
TNF bioassay
To assess nonpeptide-specific T-cell reactivities of well-characterized Vγ9/Vδ2 phosphoantigen-reactive T-cell clones,19 a TNF bioassay was performed as described previously using the ultrasensitive WEHI-164 clone 13.20The WEHI cells were grown in complete medium supplemented with 5% FCS (Gibco). All assays were calibrated with recombinant human TNF.
Patients
The Aspergillus-specific T-cell reactivities were assessed in healthy adults and in patients with clinical evidence of invasive aspergillosis after dose-intensive induction/consolidation chemotherapy for acute myeloid (n = 8) and acute lymphoblastic leukemia (n = 4), as well as in patients after allogeneic peripheral blood SCT from matched sibling donors (n = 4) and after bone marrow transplantation from a matched unrelated donor (n = 4; Table1). The median age in patients treated with chemotherapy only was 46 years (range, 29-67 years) and in patients after allogeneic SCT, 35.5 years (range, 26-48 years). Myeloablative conditioning therapy prior to allogeneic SCT consisted either of fractionated total body irradiation (12 Gy) or busulfan (16 mg/kg body weight [bw]), both in combination with cyclophosphamide (120 mg/kg bw). Patients with high-risk leukemia were additionally treated with etoposide (40 mg/kg bw) or cytosinarabinoside (2 × 2g/m2 on 2 successive days). Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporin A according to serum levels and antithymocyte globulin for 3 days at 20 mg/kg bw on days −4 to −2 in patients receiving a transplant from a matched sibling donor, or for 4 days (−4 to −1) in patients receiving a transplant from a matched unrelated donor.
To compare the immune reconstitution to fungal, bacterial, and viral antigens late after allogeneic SCT, blood samples from 18 patients taken at a median of 134 days (range, 86-468 days) after transplantation were analyzed. Patients characteristics are shown in detail in Table 2. This time point was selected, as according to our previous findings, CMV-specific lymphoproliferative responses can be demonstrated in the majority of patients at around day 100 after transplantation.18 All patients gave informed consent to donate blood for immune reconstitution studies.
Definition of invasive aspergillosis
Invasive aspergillosis was categorized according to consensus criteria recently published by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group (EORTC/IFICG) and the National Institute of Allergy and Infectious Diseases/Mycoses Study Group (NIAID/MSG).21
A diagnosis of a proven invasive aspergillosis required histologic proof of a mold infection or a positive culture (or both) obtained by a sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with infection.
Diagnosis of probable invasive aspergillosis required at least one defined host factor (neutropenia < 500/μL for > 10 days, fever for > 96 hours refractory to broad-spectrum antibiotics, signs and symptoms of GVHD, or prolonged use of corticosteroids), and at least one microbiologic (positive culture from sputum or bronchoalveolar lavage fluid, positive cytology or microscopy from a sinus aspirate) and major clinical criterion (new pulmonary infiltrates, halo sign, air-crescent sign, suggestive radiologic findings for an invasive sinus or central nervous system infection).
Possible invasive aspergillosis was defined as at least one criterion from the host section and one microbiologic or one major clinical criterion from an abnormal site consistent with infection.
According to the state of the lung infiltrates in the computed tomography (CT) scan at the time of blood sampling, patients were classified as having “regression” of invasive aspergillosis if the lesions were smaller compared to previous CT scans, “stable” if there was no difference in the extent of disease manifestations at both dates of examination, or “progression” if the manifestations became worse compared to the preceding assessment.
Statistical analysis
All observed variables were markedly nonnormally distributed and therefore described with their median and ranges. Wilcoxon signed ranks tests for dependent variables were applied to compare the stimulation indices of the best concentration of EC SAB for lymphoproliferation with the stimulation indices of the other EC SAB concentrations assessed in healthy volunteers. Mann-Whitney U tests were conducted to compare the distributions of the variables between patients with progressive clinical manifestations of invasive aspergillosis and patients with clinical response on antifungal treatment. Spearman rank order correlation coefficients were computed to describe association between Aspergillus-specific lymphoproliferation and neutrophil counts and steroid doses and between steroid doses and IFN-γ and IL-10 release in culture supernatants. The McNemar tests and sign tests were used to compare the lymphoproliferative responses after stimulation with CMV, EC SAB, and tetanus toxoid, and to compare INF-γ/IL-10 ratios. All statistical analyses in this study were done for descriptive purposes without prestated hypotheses. Test results were presented with nominal 2-tailedP values. All analyses were carried out with JMP version 3.1.6.2 and SAS system for Windows 8.0 software (SAS Institute, Cary, NC).
Results
Lymphoproliferative responses to A fumigatusantigens in healthy individuals
MNCs from healthy volunteers (n = 16) were incubated with declining concentrations of A fumigatus total antigenic extract EC SAB ranging from 50 μg to 50 ng equivalent protein/mL. A maximum SI of 3 or higher (median SI, 7.1; range, 2.1- 86.9) was documented in 14 of 16 healthy individuals (87.5%). All healthy individuals demonstrated a significant lymphoproliferation to PHA (median SI, 15.6; range, 4.1-176.5) and tetanus toxoid (median SI, 9.4; range, 3.3-191.6), and 14 of 16 individuals to IL-2 (median SI, 11; range, 2.2-132.5). Lymphocytes stimulated with 5 μg/mL EC SAB antigen demonstrated 3.3-, 1.5-, and 2.5-fold greater proliferation than did those stimulated with 50 μg/mL, 500 ng/mL, or 50 ng/mL (5 versus 50 μg, P < .0001; 5 versus 0.5 μg,P = .013; 5 versus 0.05 μg, P < .0001). On the basis of these results, we selected the 5 μg/mL concentration for all further experiments.
The lymphoproliferative capacity of CD4+, CD8+, and CD4−/CD8− T-cell subpopulations was assessed after stimulation with EC SAB in 3 individuals. In all cases, the CD4+ T-cell fraction was found to proliferate in response to EC SAB, whereas no significant lymphoproliferation was found in the CD8+ and CD4−/CD8−lymphocyte populations (data not shown).
Lymphoproliferative responses to heat-inactivated conidia were assessed in 8 healthy individuals with 7 of 8 (87.5%) demonstrating a significant lymphoproliferation (median SI, 17.45; range, 1.6-83.1). The best lymphoproliferative response was documented at a concentration of 1 × 105Aspergillusconidia/mL. As with EC SAB, the stimulation was definitely lower with the highest dose tested.
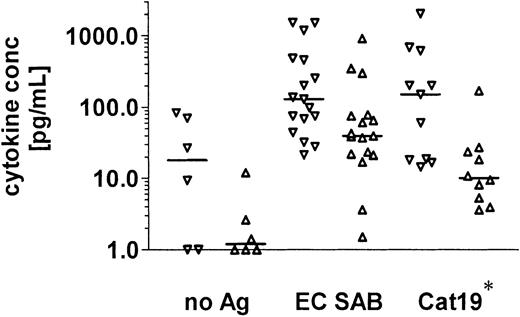
A significant lymphoproliferative response to the recombinant antigens was documented in 8 of 11 healthy individuals after stimulation with the 90-kDa catalase, and in 4 of 16 individuals in response to the 88-kDa DPP V (Figure 1).
Aspergillus -specific T-cell responses in healthy volunteers.
Aspergillus-specific T-cell responses were assessed by a lymphoproliferation assay. A stimulation index of 3 or higher was considered to indicate a positive lymphoproliferative T-cell response.Aspergillus-specific T-cell proliferation was detectable against EC SAB in 14 of 16 healthy individuals, against heat-inactivated conidia in 7 of 8, and against 2 recombinant A fumigatus proteins expressed in P pastoris, the 90-kDa catalase in 8 of 11 and the 88-kDa DPP V in 4 of 16, respectively. EC SAB indicates cellular extracts of A fumigatus; Cat19, 90-kDa catalase; and DPP, dipeptidylpeptidase.
Aspergillus -specific T-cell responses in healthy volunteers.
Aspergillus-specific T-cell responses were assessed by a lymphoproliferation assay. A stimulation index of 3 or higher was considered to indicate a positive lymphoproliferative T-cell response.Aspergillus-specific T-cell proliferation was detectable against EC SAB in 14 of 16 healthy individuals, against heat-inactivated conidia in 7 of 8, and against 2 recombinant A fumigatus proteins expressed in P pastoris, the 90-kDa catalase in 8 of 11 and the 88-kDa DPP V in 4 of 16, respectively. EC SAB indicates cellular extracts of A fumigatus; Cat19, 90-kDa catalase; and DPP, dipeptidylpeptidase.
Cytokine secretion in culture supernatants of healthy individuals
PBMNCs from 17 healthy individuals were stimulated for 5 days with the A fumigatus antigen EC SAB at a concentration of 5 μg/mL, and with the 90-kDa catalase in 11 of these 17 individuals.
The baseline IFN-γ and IL-10 release in culture supernatants after 5 days of culture without the addition of antigen was assessed in 6 of 17 healthy individuals. IFN-γ was detectable at a median concentration of 18.1 pg/mL (range, < 1.0-83.8 pg/mL) and IL-10 at a median concentration of 1.2 pg/mL (range, < 1.0-11.9 pg/mL).
After stimulation of PBMNCs with EC SAB, an increase in the production of IFN-γ (median 128.7 pg/mL; range, 21.3-1519 pg/mL) and IL-10 (median, 39.4 pg/mL; range, 1.5-899.4 pg/mL) was documented, with 13 of 17 demonstrating IFN-γ concentrations at least 2 times higher compared to IL-10. The cytokine release in culture supernatants after stimulation with the 90-kDa catalase revealed comparable results with an increased secretion of IFN-γ (n = 11; median concentration 149.3 pg/mL; range, 14.4-> 2000 pg/mL) and IL-10 (n = 10, median concentration, 10.05 pg/mL; range, 3.6-168.5 pg/mL; Figure2).
Aspergillus -specific T-cell stimulation in healthy volunteers is associated with increased production of IFN-γ and IL-10 in culture supernatants.
Cytokines were measured by standard ELISAs in the culture supernatants of PBMNCs stimulated with A fumigatus proteins. After stimulation of PBMNCs with EC SAB, an increase in the production of IFN-γ (median, 128.7 pg/mL; range, 21.3-1519 pg/mL) and IL-10 (median, 39.4 pg/mL; range, 1.5-899.4 pg/mL) was documented. The cytokine release in culture supernatants after stimulation with the 90-kDa catalase revealed comparable results (median IFN-γ concentration, 149.3 pg/mL; range, 14.4-> 2000 pg/mL; median IL-10 concentration, 10.05 pg/mL; range, 3.6-168.5 pg/mL). EC SAB indicates cellular extracts of A fumigatus; Cat19, 90-kDa catalase; ▿, IFN-γ; ▵, IL-10; and *, IL-10 concentrations were assessed in 10 of 11 healthy individuals.
Aspergillus -specific T-cell stimulation in healthy volunteers is associated with increased production of IFN-γ and IL-10 in culture supernatants.
Cytokines were measured by standard ELISAs in the culture supernatants of PBMNCs stimulated with A fumigatus proteins. After stimulation of PBMNCs with EC SAB, an increase in the production of IFN-γ (median, 128.7 pg/mL; range, 21.3-1519 pg/mL) and IL-10 (median, 39.4 pg/mL; range, 1.5-899.4 pg/mL) was documented. The cytokine release in culture supernatants after stimulation with the 90-kDa catalase revealed comparable results (median IFN-γ concentration, 149.3 pg/mL; range, 14.4-> 2000 pg/mL; median IL-10 concentration, 10.05 pg/mL; range, 3.6-168.5 pg/mL). EC SAB indicates cellular extracts of A fumigatus; Cat19, 90-kDa catalase; ▿, IFN-γ; ▵, IL-10; and *, IL-10 concentrations were assessed in 10 of 11 healthy individuals.
Vγ9/Vδ2 T-cell clones are reactive to A fumigatus antigens
The Aspergillus-specific responses of well-characterized Vγ9/Vδ2 T-cell clones were assessed by a TNF bioassay. In 6 independent experiments, well-characterized phosphoantigen-reactive Vγ9/Vδ2 T-cell clones, but not control α/β and control phosphoantigen-nonreactive γ/δ T-cell clones, produced significant quantities of TNF (20-40 pg/mL) to the EC SAB antigen (dilution 1:40), suggesting that Aspergillus antigen preparations may contain nonpeptidic antigens (Figure3).
Phosphoantigen-reactive Vγ9/Vδ2 T-cell clones, but not α/β, and phosphoantigen-nonreactive γ/δ T-cell clones, produce TNF in response to A fumigatus antigens.
Aspergillus-specific T-cell responses were assessed by a TNF bioassay using the ultrasensitive WEHI-164 clone 13. The phosphoantigen-reactive Vγ9/Vδ2 T-cell clones C49 and 6E1, but not the phosphoantigen-nonreactive γ/δ T-cell clone AB18 and the αβ T-cell clone B1, produced significant amounts of TNF in response toA fumigatus antigen EC SAB.
Phosphoantigen-reactive Vγ9/Vδ2 T-cell clones, but not α/β, and phosphoantigen-nonreactive γ/δ T-cell clones, produce TNF in response to A fumigatus antigens.
Aspergillus-specific T-cell responses were assessed by a TNF bioassay using the ultrasensitive WEHI-164 clone 13. The phosphoantigen-reactive Vγ9/Vδ2 T-cell clones C49 and 6E1, but not the phosphoantigen-nonreactive γ/δ T-cell clone AB18 and the αβ T-cell clone B1, produced significant amounts of TNF in response toA fumigatus antigen EC SAB.
Aspergillus-specific T-cell responses in patients with clinical evidence of invasive aspergillosis
According to the above-mentioned definitions, 5 patients suffered from proven, 3 from probable, and 12 from possible invasive aspergillosis.
After stimulation with the whole A fumigatus antigen extract EC SAB, a positive lymphoproliferative response was documented in 14 of 18 patients (median SI, 9.05; range, 1.0-41.4; Table 1). Two additional patients (nos. 7 and 20) not tested with EC SAB showed a positive lymphoproliferative response to an ethanol precipitate of a culture filtrate of A fumigatus (PP EXL) grown in a culture medium containing 1% yeast extract (Difco; SI 3.6 and 13.2, respectively). A positive lymphoproliferative response to PHA was documented in 14 of these 20 patients (median SI, 18.75; range, 0.6-332.1), to tetanus toxoid in 10 of 18 analyzed (median SI, 3.6; range, 0.7-27.2), and to IL-2 in 17 of 20 patients (median SI, 15.95; range, 0.5-201.5).
Proven or probable invasive aspergillosis was diagnosed in 5 patients after allogeneic SCT (nos. 1, 2, 6, 7, and 8), and in 3 further patients following intensive chemotherapy for acute myeloid leukemia (nos. 3, 4, and 5) the invasive aspergillosis was proven (Table 1). Six of 7 patients showed a positive lymphoproliferative response to EC SAB and patient no. 7 to the ethanol precipitate. Five of these 8 patients demonstrated at least a partial regression of clinical manifestations of invasive aspergillosis, and patient no. 6 after allogeneic SCT survived cerebral aspergillosis for more than 6 weeks (Table 1). After initial improvement and control of invasive aspergillosis, patients nos. 4 and 5 underwent an allogeneic SCT and subsequently died from disseminated invasive aspergillosis. Another 12 patients with possible invasive aspergillosis according to the above-mentioned definitions were analyzed and 8 of 11 showed an SI more than or equal to 3 in response to EC SAB and patient no. 20 after stimulation with the ethanol precipitate (Table 1). A low SI in response to the EC SAB antigen was demonstrated in 3 of 6 patients after allogeneic SCT with progressive disease (Table 1, patients nos. 9, 10, and 11).
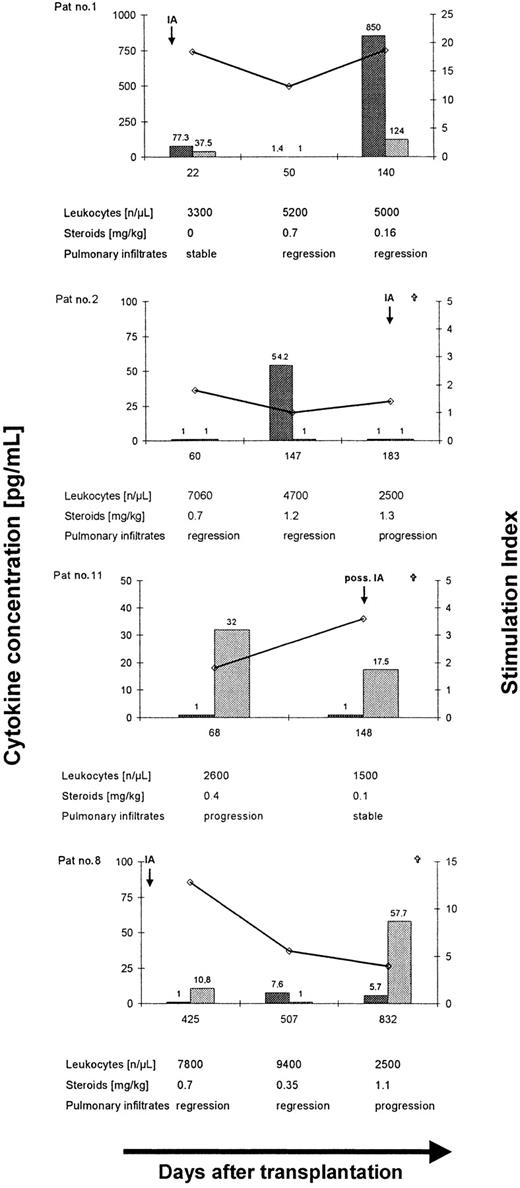
The release of IFN-γ and IL-10 in culture supernatants after stimulation with EC SAB was assessed in 17 patients. Baseline cytokine release in culture supernatants after 5 days of culture assessed in 6 patients demonstrated low concentrations of IFN-γ (median, 2.4; range, < 1.0-20.8 pg/mL) and IL-10 (median, 1.6; range, < 1.0-11.9 pg/mL; median ratio IFN-γ/IL-10 = 0.9; range, 0.2-21). A higher ratio of IFN-γ/IL-10 was documented in patients with favorable response to antifungal therapy (n = 7, median ratio, IFN-γ/IL-10 = 1.0; range, 0.09-24.8) compared to 10 patients with progressive (n = 6) or stable (n = 4) disease (median ratio, IFN-γ/IL-10 = 0.1; range, 0.002-2.1; P = .04). Interestingly, 2 of the 7 patients who demonstrated a favorable response to antifungal therapy at the time of analysis despite a lowAspergillus-specific lymphoproliferation or a low IFN-γ/IL-10 ratio (Table 1, patients no. 2 and 8; Figure4), finally died from disseminated invasive aspergillosis within 3 to 14 months. Both patients received prolonged immunosuppression following allogeneic SCT. Thus, control of invasive aspergillosis was found to be correlated with a TH1 response (Figure 4, patient no. 1), whereas fatal outcome of the infection was associated with a low SI (Figure 4, patient no. 2) and a TH2 cytokine pattern (Figure 4, patients no. 8 and 11).
Longitudinal analysis ofAspergillus-specific T-cell responses in 4 patients with invasive aspergillosis after allogeneic SCT.
Long-term control of invasive aspergillosis was associated with a lymphoproliferative response to EC SAB and a dominant release of IFN-γ (patient no. 1), whereas a low SI (patient no. 2) or a dominant release of IL-10 (patients no. 8 and 11) was found to be associated with disease dissemination and fatal outcome of invasive aspergillosis after allogeneic SCT. ⋄, stimulation index; , IFN-γ; and
, IFN-γ; and , IL-10.
, IL-10.
Longitudinal analysis ofAspergillus-specific T-cell responses in 4 patients with invasive aspergillosis after allogeneic SCT.
Long-term control of invasive aspergillosis was associated with a lymphoproliferative response to EC SAB and a dominant release of IFN-γ (patient no. 1), whereas a low SI (patient no. 2) or a dominant release of IL-10 (patients no. 8 and 11) was found to be associated with disease dissemination and fatal outcome of invasive aspergillosis after allogeneic SCT. ⋄, stimulation index; , IFN-γ; and
, IFN-γ; and , IL-10.
, IL-10.
The correlation of the clinical response at the time of analysis revealed Aspergillus-specific lymphoproliferation to be correlated with the neutrophil count (R = 0.459;P = .064) and to be inversely correlated with the steroid dose (R = −0.508; P = .037). Moreover, steroid treatment was associated with suppression of IFN-γ (R = 0.572; P = .017) but not IL-10 release (R = −0.2543; P = .325) in culture supernatants.
Comparative analysis of lymphoproliferative responses toA fumigatus, tetanus toxoid, and CMV in patients late after allogeneic SCT
To compare the immune reconstitution to fungal, bacterial, and viral antigens in patients after allogeneic SCT, blood samples from 18 patients taken at a median of 134 days (range, 86-468 days) after SCT were analyzed. This time point was selected because, according to our previous results, CMV-specific T-cell reconstitution can be demonstrated in the majority of patients at around day 100 after transplantation.
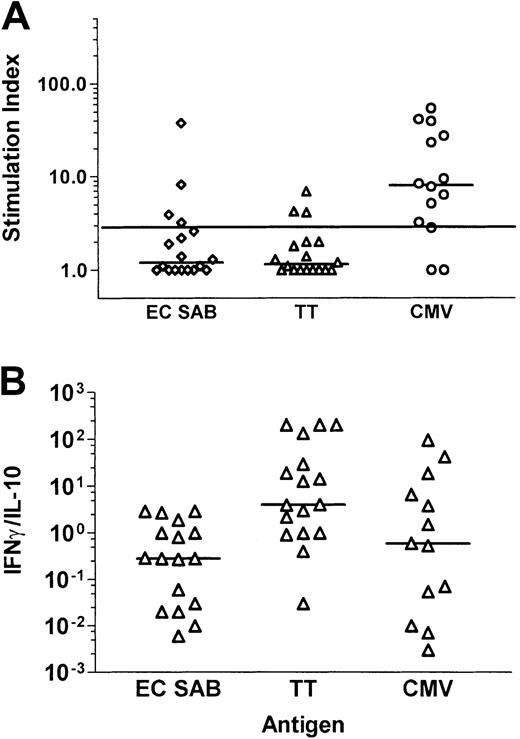
A positive lymphoproliferative response was documented in 11 of 14 patients—tested CMV seropositive before transplantation or receiving a transplant from a CMV-seropositive donor—after stimulation with CMV antigen (median SI, 8.0; range, 1.0-53.8), compared to 4 of 18 patients after stimulation with the A fumigatus antigen EC SAB (median SI, 1.2; range, 1.0-37.6; P = .008, McNemar test), and to 3 of 18 after stimulation with tetanus toxoid (median SI, 1.15; range ,1.0-6.9; P = .003, McNemar test; Figure5A). No lymphoproliferative response to CMV antigen was detectable in 4 CMV-seronegative patients receiving a transplant from a CMV-seronegative donor (data not shown). Interestingly, 3 of the 4 patients demonstrating anAspergillus-specific lymphoproliferation suffered from invasive aspergillosis after transplantation (n = 2) or had a positive history of invasive aspergillosis before transplantation (n = 1).
Comparative analysis of Aspergillus-, tetanus toxoid–, and CMV antigen–specific T-cell responses in patients late after allogeneic SCT.
Cytokines were measured by standard ELISAs in the culture supernatants of PBMNCs stimulated with A fumigatus antigen EC SAB. Panel A shows the stimulation indices, and panel B the ratio of IFN-γ and IL-10. An SI of 3 or higher was considered to indicate a positive lymphoproliferative T-cell response. Aspergillus-specific T-cell responses in patients late after allogeneic SCT were characterized by a low SI and a low IFN-γ/IL-10 ratio.
Comparative analysis of Aspergillus-, tetanus toxoid–, and CMV antigen–specific T-cell responses in patients late after allogeneic SCT.
Cytokines were measured by standard ELISAs in the culture supernatants of PBMNCs stimulated with A fumigatus antigen EC SAB. Panel A shows the stimulation indices, and panel B the ratio of IFN-γ and IL-10. An SI of 3 or higher was considered to indicate a positive lymphoproliferative T-cell response. Aspergillus-specific T-cell responses in patients late after allogeneic SCT were characterized by a low SI and a low IFN-γ/IL-10 ratio.
The cytokine concentrations of IFN-γ and IL-10 were analyzed after stimulation with EC SAB and tetanus toxoid in 17 patients and after stimulation with CMV antigen in 13 patients. The median IFN-γ/IL-10 ratio in response to tetanus toxoid (median ratio, 4.04; range, 0.026-202) was higher compared to the stimulation with EC SAB (median ratio, 0.28; range, 0.006-2.93; P = .002, sign test) and CMV antigen (median ratio, 0.58; range, 0.003-95.24;P = .267, sign test; Figure 5B). Thus, theAspergillus-specific T-cell response in patients late after allogeneic SCT was characterized by a low SI and a low IFN-γ/IL-10 ratio, indicating a TH2 response in some of the patients, potentially contributing to the prolonged risk for the development of invasive aspergillosis in this patient group.
Discussion
In the last 1 to 2 decades, an important change in the epidemiology of invasive aspergillosis was observed. Whereas in earlier years, this devastating disease was almost always observed in hematologic patients with long-lasting neutropenia, invasive aspergillosis was reported more recently with an increasing frequency in nonneutropenic patients after allogeneic SCT,1 in patients with advanced HIV infection,6 and in critically ill neonates.7 Transmission via airborne spores was identified as the major route of infection.22 In some patients, colonization occurs without the development of invasive disease; in others, the infection remains restricted to the lungs or may disseminate to a variety of organs and tissues, and on postmortem analysis, Aspergillus may be documented in almost all tissues.23
The pathophysiology of invasive aspergillosis, especially in nonneutropenic patients, is not completely understood. Recognized risk factors for invasive aspergillosis are defects in phagocyte function,2 corticosteroid-induced suppression of phagocyte function,3,24 and long-lasting neutropenia.4More recently, an increased incidence of invasive fungal infections was observed in patients after allogeneic bone marrow transplantation compared to peripheral blood SCT.25 The only difference between both groups was a faster T-cell reconstitution after transplantation of peripheral blood stem cells indicating a potential role of T cells for the control of fungal infections.
Dysregulation of cytokine release has been identified as an important risk factor in patients with HIV infection.26 In the murine model of invasive pulmonary aspergillosis, resistance to infection was associated with IFN-γ–producing interstitial lung lymphocytes and TNF-α10,12,13 and IL-12 production,12 whereas a dominant release of TH2 cytokines of interstitial lung lymphocytes was associated with progressive disease. TH1-mediated resistance to invasive aspergillosis was further confirmed by neutralization of TH2 cytokines and in IL-4 and IL-10 knockout animals.12,27 Protective immunity was documented in animals sensitized with a sublethal challenge of A fumigatus conidia developing a TH1 response on subsequent exposure to lethal infection.10,12 Treatment of immunocompetent mice with Aspergillus crude culture filtrate antigens resulted in the development of local and peripheral protective TH1 memory responses, mediated byAspergillus-specific CD4+ T cells producing IFN-γ and IL-2 capable of conferring protection on adoptive transfer to naı̈ve recipients.15
Based on these clinical observations and experimental results,Aspergillus-specific T-cell responses were assessed in healthy individuals, immunosuppressed patients with clinical evidence of invasive aspergillosis, and nonneutropenic patients late (> 100 days) after allogeneic SCT. Almost all healthy individuals demonstrated a significant lymphoproliferation to cellular extracts of A fumigatus, heat-inactivated A fumigatus conidia as reported before,28 and to the 2 major antigens of A fumigatus, the 90-kDa catalase and the 88- kDa DPP V. Assessment of the IFN-γ and IL-10 concentrations in culture supernatants after specific stimulation suggested a TH1 type of immune response. Patients with clinical evidence of invasive aspergillosis and disease regression on antifungal therapy were characterized by positiveAspergillus-specific lymphoproliferation and a higher ratio of IFN-γ/IL10 in culture supernatants after specific stimulation with cellular extracts of A fumigatus compared to patients with stable or progressive disease demonstrating a lower IFN-γ/IL10 ratio in culture supernatants (P = .04). Interestingly, 2 of the 7 patients who initially demonstrated a favorable response to antifungal therapy despite a low Aspergillus-specific lymphoproliferation or a low IFN-γ/IL-10 ratio finally died from disseminated invasive aspergillosis. Both patients had received prolonged immunosuppression after allogeneic SCT. Thus, in line with the results of the murine model of invasive aspergillosis, our data further support a potential role of a TH1 immune response for the successful control of invasive aspergillosis in patients with hematologic malignancies.
Major risk factors for invasive aspergillosis after allogeneic SCT are prolonged neutropenia, acute and chronic GVHD, and corticosteroid treatment.1 In our study, a low lymphoproliferative response to A fumigatus antigens was found to be associated with corticosteroid treatment, and a low release of IFN-γ in culture supernatants on specific stimulation. Patients with progressive disease despite a positive lymphoproliferative response toAspergillus antigens either demonstrated a low ratio of IFN-γ/IL-10 in culture supernatants or very low levels of both cytokines. Thus, we conclude from this analysis suppression ofAspergillus-specific TH1 response after allogeneic SCT during GVHD and corticosteroid treatment to be a predisposing factor for the acquisition and progression of invasive aspergillosis. In line with these results, nonneutropenic patients with favorable outcome demonstrated low IL-10 levels, whereas progressive disease was associated with elevated IL-10 levels.29Moreover, we found Aspergillus-specific T-cell responses to be characterized by a low SI and a low IFN-γ/IL-10 ratio in many patients late after allogeneic SCT, potentially contributing to the prolonged risk for the development of invasive aspergillosis in this patient group.1
In addition to T-helper cell responses, the release of TNF from γ/δ T-cell clones from healthy individuals in response to A fumigatus was studied. Phosphoantigen-reactive Vγ9/Vδ2 T-cell clones, but not control α/β, and control phosphoantigen-nonreactive γ/δ T-cell clones, produced significant quantities of TNF to the EC SAB antigen. These data indicate that Aspergillus antigen preparations may contain nonpeptidic antigens for Vγ9/Vδ2 T cells.30-32 To our knowledge, aspergillus antigens have not been described up to now to stimulate γ/δ T-cells. Our data suggest that Vγ9/Vδ2 T cells may contribute to the protective immune response against invasive aspergillosis. Early expansion of CD45RO+ Vγ9/Vδ2 T cells during the first weeks after transplantation, possibly on contact with environmental antigens, has been reported in patients after allogeneic SCT,33 and supranormal levels of γ/δ T cells were found to be associated with infectious complications after allogeneic SCT.34 Thus, γ/δ T cells may function as a bridge between innate and adaptive immunity in the defense against fungal infections.35
In conclusion, the data reported support the hypothesis that in patients with hematologic malignancies, T cells may contribute to the host defense against A fumigatus. In the future, a detailed analysis of Aspergillus-specific T-cell responses may help to develop new antifungal treatment strategies, such as treatment with proinflammatory cytokines, inhibition of IL-10, or the adoptive transfer of Aspergillus-specific TH1 cells in patients after allogeneic SCT.
We thank Prof Dr Hans-Georg Rammensee and Prof Dr Lothar Kanz for helpful discussions and Friederike Frank for technical assistance.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-01-0265.
Supported by grants from the Federal Ministry of Education and Research (Fö. 01KS9602) and the Interdisciplinary Center of Clinical Research Tübingen (IZKF), project IIC7, and the AG Infektionen in Hämatologie und Onkologie of the Deutsche Gesellschaft für Hämatologie und Onkologie (DGHO).
H.H. and C.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Holger Hebart, Medizinische Klinik II, Otfried-Müller Str 10, D-72076, Tübingen, Germany; e-mail:hrhebart@med.uni-tuebingen.de.