Abstract
We previously demonstrated that 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311) and other aroylhydrazone chelators possess potent antineoplastic activity because of their ability to bind iron (Fe). From these studies, we identified structural components of the hydrazones that provide antineoplastic activity, namely the salicylaldehyde and 2-hydroxy-1-naphthylaldehyde moieties. A related group of chelators known as the thiosemicarbazones also show pronounced antitumor activity because of their ability to inhibit ribonucleotide reductase. Considering this, we designed a new series of “hybrid ligands” by condensation of the aldehydes described above with a range of thiosemicarbazides. The parent compound of these ligands is 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT). Of 8 NT analogues, 3 chelators, namely NT, N4mT (2-hydroxy-1-naphthylaldehyde-4-methyl-3-thiosemicarbazone), and N44mT (2-hydroxy-1-naphthylaldehyde-4,4-dimethyl-3-thiosemicarbazone), showed high antiproliferative activity against SK-N-MC neuroepithelioma cells (50% inhibitory concentration [IC50] = 0.5-1.5 μM). Indeed, their activity was significantly (P < .0001) greater than that of desferrioxamine (DFO) (IC50 = 22 μM). We demonstrate that 311, a 311 analogue (311m), and several NT-series chelators have significantly (P < .001) greater antiproliferative activity against tumor cells than against a range of normal cell types. For example, the IC50 values of NT and N4mT in SK-N-MC neuroepithelioma cells were 0.5 μM, whereas for fibroblasts the IC50 values were greater than 25 μM. Further, the effect of one of the most potent chelators (311m) on preventing the growth of bone marrow stem cell cultures was far less than that of doxorubicin and similar to that of cisplatin. These studies support the further development of these chelators as antiproliferative agents.
Introduction
Iron (Fe) plays a critical role in a variety of metabolic processes, as Fe-containing proteins catalyze key reactions involved in energy production and DNA synthesis (eg, ribonucleotide reductase [RR]).1,2 In the absence of Fe, cells cannot proceed from the G1 to the S phase of the cell cycle.1-4 Further, RR is the rate-limiting step in DNA synthesis, and the fact that Fe is critical for RR activity means it is an important target for antitumor drugs.5-7
Neuroblastoma (NB) is an aggressive childhood tumor with a poor prognosis, and new therapies are urgently required.8Interestingly, NB cells from patients with advanced disease contain increased amounts of ferritin rich in Fe.9,10 Evidence of a sensitive relationship between Fe and NB cell growth is suggested by the observation that the chelator desferrioxamine (DFO) is capable of a cytotoxic effect on NB cells in vitro while having little effect on other cells.11,12 Indeed, clinical trials with DFO in patients with NB and leukemia have shown significant results.13-17 Despite the link between Fe metabolism and the antineoplastic effect of DFO and other Fe chelators,2little is known about the molecular targets of Fe chelators or the structure–activity relationships involved.
We characterized chelators known as the pyridoxal isonicotinoyl hydrazone (PIH) analogues.18-24 Some of these (eg, 311) show much higher antiproliferative activity against tumor cells than DFO.21,24-27 After screening 36 PIH analogues, we identified structural characteristics of these Fe chelators that result in antiproliferative activity.21,24 We found that analogues derived from pyridoxal possess high Fe-chelation activity and low antiproliferative effects, properties of ligands suitable to treat Fe overload.21 In contrast, analogues derived from salicylaldehyde and particularly 2-hydroxy-1-naphthylaldehyde possess pronounced antiproliferative activity plus high Fe-chelation efficacy, characteristics of ligands suitable to treat neoplasia.21 24
Intriguingly, a series of chelators related to PIH known as the thiosemicarbazones (eg, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, Triapine; Vion Pharmaceuticals Inc, New Haven, CT) have been described as the most effective inhibitors of RR yet identified.28-31 We showed that the 2-pyridyl component of these latter ligands does not confer antineoplastic activity on our PIH analogues.32Considering this, we believe that the other half of the molecule, that is, the thiosemicarbazide moiety (NH2-CS-NH-NH2), could mediate antiproliferative activity. Therefore, condensation of 2-hydroxy-1-naphthylaldehyde with thiosemicarbazide to form 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT; Figure1) may result in a highly active chelator.
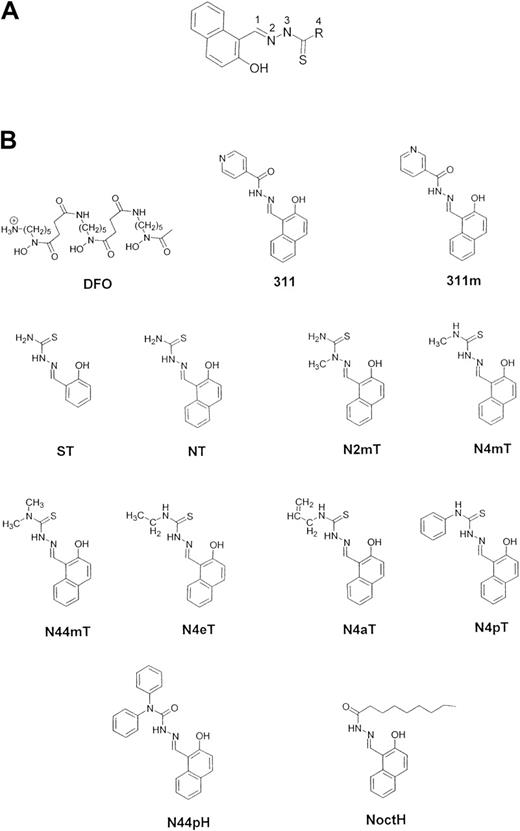
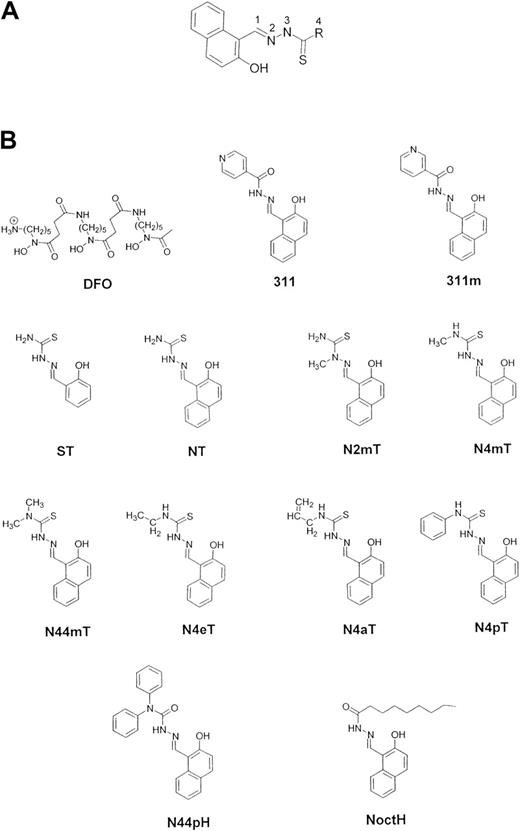
Structures of the Fe chelators examined in this study.
(A) General structure of the 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT) analogues along with their numbering scheme. (B) Desferrioxamine (DFO), 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311), 2-hydroxy-1-naphthylaldehyde nicotinoyl hydrazone (311m), salicylaldehyde thiosemicarbazone (ST), 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT), 2-hydroxy-1-naphthylaldehyde-2-methyl-3-thiosemicarbazone (N2mT), 2-hydroxy-1-naphthylaldehyde-4-methyl-3-thiosemicarbazone (N4mT), 2-hydroxy-1-naphthylaldehyde-4,4-dimethyl-3-thiosemicarbazone (N44mT), 2-hydroxy-1-naphthylaldehyde-4-ethyl-3-thiosemicarbazone (N4eT), 2-hydroxy-1-naphthylaldehyde-4-allyl-3-thiosemicarbazone (N4aT), 2-hydroxy-1-naphthylaldehyde-4-phenyl-3-thiosemicarbazone (N4pT), 2-hydroxy-1-naphthylaldehyde-4,4-diphenylhydrazone (N44pH), and 2-hydroxy-1-naphthylaldehyde-4-octylhydrazone (NoctH).
Structures of the Fe chelators examined in this study.
(A) General structure of the 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT) analogues along with their numbering scheme. (B) Desferrioxamine (DFO), 2-hydroxy-1-naphthylaldehyde isonicotinoyl hydrazone (311), 2-hydroxy-1-naphthylaldehyde nicotinoyl hydrazone (311m), salicylaldehyde thiosemicarbazone (ST), 2-hydroxy-1-naphthylaldehyde thiosemicarbazone (NT), 2-hydroxy-1-naphthylaldehyde-2-methyl-3-thiosemicarbazone (N2mT), 2-hydroxy-1-naphthylaldehyde-4-methyl-3-thiosemicarbazone (N4mT), 2-hydroxy-1-naphthylaldehyde-4,4-dimethyl-3-thiosemicarbazone (N44mT), 2-hydroxy-1-naphthylaldehyde-4-ethyl-3-thiosemicarbazone (N4eT), 2-hydroxy-1-naphthylaldehyde-4-allyl-3-thiosemicarbazone (N4aT), 2-hydroxy-1-naphthylaldehyde-4-phenyl-3-thiosemicarbazone (N4pT), 2-hydroxy-1-naphthylaldehyde-4,4-diphenylhydrazone (N44pH), and 2-hydroxy-1-naphthylaldehyde-4-octylhydrazone (NoctH).
In this study, we designed a novel class of NT analogues that incorporate features of the thiosemicarbazones into the structural components of the most effective PIH analogues identified.21,24 Hence, we condensed salicylaldehyde or 2-hydroxy-1-naphthylaldehyde with a range of thiosemicarbazides, resulting in chelators with a wide range of lipophilicities. It was important to vary this latter factor because of its relationship to Fe-chelation efficacy and antiproliferative activity.19,21,33 34
The characterization of novel ligands is important in terms of obtaining the patent protection necessary for development by the pharmaceutical industry. Previous studies with 311 were reported without patent protection,21 24 and this obstructed its development. Hence, in addition to the NT analogues, we also assessed the activity of 3 new 311 analogues, known as 2-hydroxy-1-naphthylaldehyde nicotinoyl hydrazone (311m), 2-hydroxy-1-naphthylaldehyde 4,4-diphenylhydrazone (N44pH), and 2-hydroxy-1-naphthylaldehyde octylhydrazone (NoctH) (Figure 1B).
We show that the combination of 2-hydroxy-1-naphthylaldehyde with various thiosemicarbazides results in novel tridentate “hybrid chelators” known as the NT series, which have significantly greater antiproliferative activity than DFO. Importantly, the antiproliferative activity is relatively selective for tumor cells, the mechanism of action being due to the chelation of intracellular Fe. Interestingly, the Fe complexes of NT and N4mT appeared to have some redox activity, although this is not as marked as that observed for other thiosemicarbazones. In summary, we have identified 3 novel hybrid chelators (NT, N4mT, and N44mT) and a 311 analogue (311m) that warrant further investigation as suitable candidates for cancer chemotherapy.
Materials and methods
Synthesis and characterization of iron chelators and their preparation for screening in culture
Cell culture
Human K562 erythroleukemia cells, SK-Mel-28 melanoma cells, SK-N-MC neuroepithelioma cells, MRC5 fibroblasts, and MCF-7 breast cancer cells were from the American Type Culture Collection (Rockville, MD). Primary cultures of human umbilical vein endothelial cells (HUVECs) and human monocyte–derived macrophages were prepared using standard techniques.37,38 Bone marrow stem cell cultures were prepared using established methods39 to assess the effects of chelators on the growth of granulocyte–macrophage (GM) colonies. The SK-N-MC line was used in most studies because the effects of chelators on these cells have been well characterized.20,21,24-27 The cells were grown using standard conditions.21
Preparation of 59Fe-transferrin
Effect of chelators on cellular proliferation and [3H]thymidine incorporation
Iron uptake and iron efflux experiments
Effect of the chelators at binding 59Fe from59Fe-transferrin
The ability of the chelators to directly remove 59Fe from 59Fe-Tf was analyzed by dialysis studies.19
Northern and Western blot analyses
Ascorbate oxidation and benzoate hydroxylation assay
Measurement of DNA integrity using the plasmid pGEM-7Zf(±) in the presence of Fe and the chelator
Escherichia coli (DH-5α) was transformed with pGEM-7Zf(+) (Promega, Madison, WI) and grown in LB medium. Plasmid DNA was then purified using the Qiagen plasmid purification kit (Qiagen, Hilden, Germany). Reagents were added in the following order: purified sterile water, chelator (1, 10, and 30 μM), FeSO4 (10 μM), H2O2 (1 mM), and plasmid DNA (10 μg/mL).45 Samples were incubated at room temperature for 30 minutes and then loaded onto a 1% agarose gel.45
For the ascorbate oxidation, benzoate hydroxylation, and plasmid DNA strand-break assays, we used the term “iron-binding equivalents” (IBEs) to express our data. We did this because of the different coordination modes of the ligands to Fe (ie, DFO and EDTA are hexadentate and form 1:1 ligand–Fe complexes, whereas 311, Triapine, and NT and its analogues are tridentate, resulting in 2:1 ligand–Fe complexes). In this study, a range of ligand–Fe IBE ratios were used, namely 0.1, 1, or 3. An IBE of 1 is equivalent to the complete filling of the coordination shell of the Fe atom by the ligand(s). Thus, for a hexadentate chelator (ie, DFO or EDTA), an IBE ratio of 1 represents 1 ligand to 1 Fe atom, whereas for a tridentate chelator (ie, Triapine, NT, or 311), it is equal to 2 ligands to 1 Fe atom. An IBE of 0.1 represents an excess of Fe to chelator (ie, 1 hexadentate chelator or 2 tridentate chelators in the presence of 10 Fe atoms). An IBE of 3 represents an excess of chelator to Fe and is equal to 3 hexadentate chelators or 6 tridentate chelators in the presence of 1 Fe atom.
Statistics
Data were compared using Student paired t test. Results were considered statistically significant whenP < .05.
Results
The effect of the chelators on the proliferation of neoplastic cells
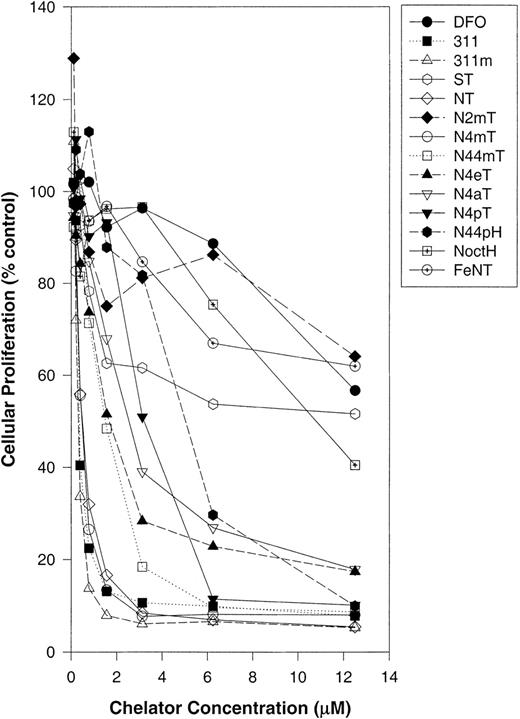
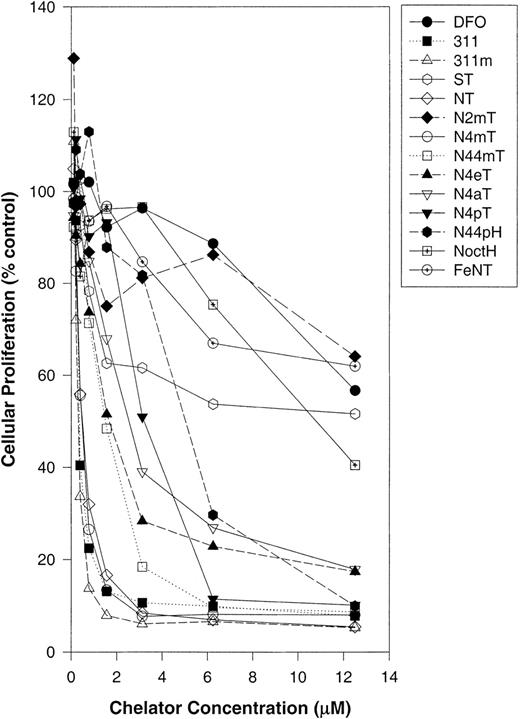
Initially, the ability of the ligands to inhibit proliferation was assessed in SK-N-MC neuroepithelioma cells (Figure2; Table1). These studies identified 311m, NT, and N4mT as having very high antiproliferative activity (50% inhibitory concentration [IC50] = 0.3-0.5 μM; Table1). These ligands were significantly (P < .0001) more active than DFO (IC50 = 22 μM) and had efficacy comparable to 311 (IC50 = 0.3 μM; Table 1). Like the Fe complexes of DFO and 311,25 26 the NT–Fe complex was not very active at inhibiting SK-N-MC cell proliferation (IC50 > 12.5 μM; Figure 2; Table 1). This suggests that the metal ion binding activity of this ligand plays a role in its activity. Other chelators with appreciable activity against SK-N-MC cells included N44mT and N4eT, which had IC50 values of 1.5 and 1.6 μM, respectively (Table 1).
The effect of chelator concentration on the proliferation of SK-N-MC neuroepithelioma cells.
Cells were incubated in the presence and absence of the chelators (0-12.5 μM) for 72 hours at 37°C. After this incubation period, cellular density was measured with the MTT assay (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
The effect of chelator concentration on the proliferation of SK-N-MC neuroepithelioma cells.
Cells were incubated in the presence and absence of the chelators (0-12.5 μM) for 72 hours at 37°C. After this incubation period, cellular density was measured with the MTT assay (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
To investigate the spectrum of antineoplastic activity, we assessed the most active analogues against SK-N-MC cells (311m, NT, N4mT, and N44mT), K562 erythroleukemia cells, SK-Mel-28 melanoma cells, and MCF-7 breast cancer cell lines (Table 1). These ligands were far more active than DFO at inhibiting proliferation of K562 and MCF-7 cells (Table 1). Against SK-N-MC cells, NT and N4mT were more active (IC50 = 0.5 μM) than N44mT (IC50 = 1.5 μM; Table 1). However, of these chelators, N44mT was most active against K562, SK-Mel-28, and MCF-7 cells (Table 1). Significantly, 311m demonstrated marked activity against all neoplastic cells (Table 1).
The chelators have less effect on the proliferation of normal cell types than neoplastic cells
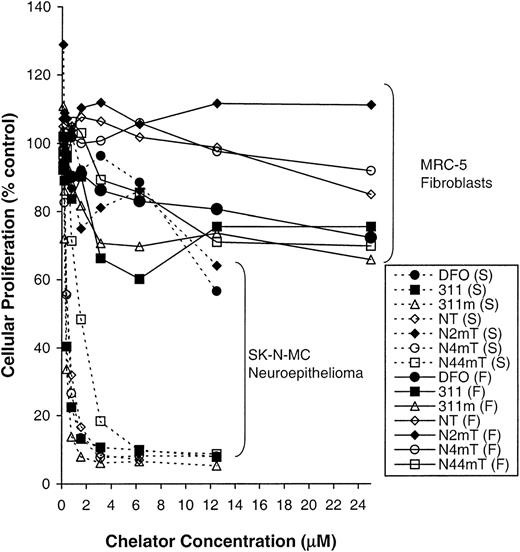
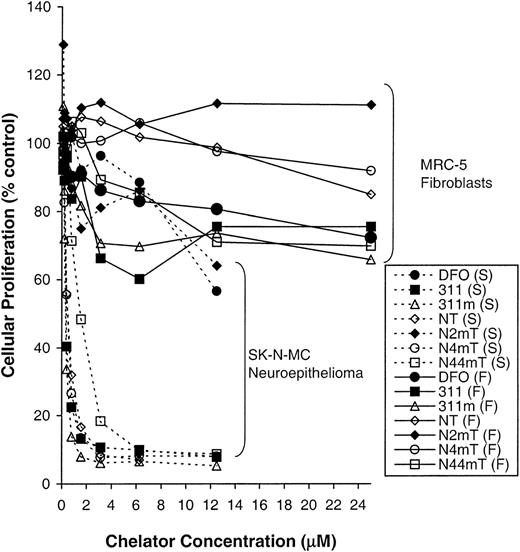
Clinically useful antitumor agents have a significant therapeutic index (ie, they have little effect on normal cells while inhibiting neoplastic cell growth). Hence, it was important to compare the antiproliferative effects of the most active chelators (311, 311m, NT, N4mT, and N44mT) between a range of neoplastic and normal cell types (Figure 3; Table 1). In these experiments, N2mT was included as a negative control because the methyl group at the 2-position (Figure 1) hinders electron delocalization and metal binding. These studies showed that in contrast to SK-N-MC cells, in which 311, 311m, NT, N4mT, and N44mT resulted in a potent antiproliferative effect (IC50 = 0.3-1.5 μM), these chelators had relatively little effect on fibroblast proliferation, with the IC50 values being greater than 25 μM (Figure 3; Table 1). The difference in the antitumor effect may be due to the lower rate of proliferation of the MRC-5 fibroblasts (doubling time, 22 hours) compared with the SK-N-MC neuroepithelioma cells (doubling time, 16 hours). For the most active chelators examined (311, 311m, NT, N4mT, and N44mT), their ability to inhibit growth was most pronounced for the neoplastic cells as compared with normal cell types. Of the normal cells examined, MRC-5 fibroblasts were the least sensitive to the action of the 5 chelators, whereas the more rapidly proliferating HUVEC cultures were the most sensitive (Table 1). Despite the similar replicative rates of HUVEC and SK-N-MC cells, the latter tumor cells were more sensitive to chelators. Comparing the 4 neoplastic cell types assessed, we found that SK-N-MC neuroepithelioma cells were the most sensitive and MCF-7 breast cancer cells were least affected.
Antiproliferative effects of the most active chelators in neoplastic and normal cells.
The influence of the most cytotoxic NT analogues, 311m, and 311 (internal standard) on the proliferation of SK-N-MC neuroepithelioma cells (S) as compared with MRC-5 fibroblasts (F). Cells were incubated in the presence and absence of the chelators (0-25 μM) for 72 hours at 37°C. After this incubation period, cellular density was measured with the MTT assay (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
Antiproliferative effects of the most active chelators in neoplastic and normal cells.
The influence of the most cytotoxic NT analogues, 311m, and 311 (internal standard) on the proliferation of SK-N-MC neuroepithelioma cells (S) as compared with MRC-5 fibroblasts (F). Cells were incubated in the presence and absence of the chelators (0-25 μM) for 72 hours at 37°C. After this incubation period, cellular density was measured with the MTT assay (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
Further studies examined the effect of one of the most potent antiproliferative agents identified in this study, namely 311m, on the proliferation of GM colonies of normal human bone marrow over 14 days. As relevant controls, the effects of the cytotoxic drugs cisplatin and doxorubicin were compared with 311m (Figure4). The ability of 311m to inhibit proliferation of GM colonies was far less marked than that of doxorubicin and similar to that of cisplatin (Figure 4).
Effect of 311m on proliferation of GM colonies of normal bone marrow.
The highly effective Fe chelator and antiproliferative agent, 311m, has similar activity to the clinically used antitumor agent cisplatin but far less activity than doxorubicin at inhibiting the growth of normal GM stem cell colonies from human bone marrow. Normal bone marrow stem cells were incubated for 14 days at 37°C with the agents (0.005-0.4 μM), and the colonies were then counted. Results are from a typical experiment.
Effect of 311m on proliferation of GM colonies of normal bone marrow.
The highly effective Fe chelator and antiproliferative agent, 311m, has similar activity to the clinically used antitumor agent cisplatin but far less activity than doxorubicin at inhibiting the growth of normal GM stem cell colonies from human bone marrow. Normal bone marrow stem cells were incubated for 14 days at 37°C with the agents (0.005-0.4 μM), and the colonies were then counted. Results are from a typical experiment.
The relationship between lipophilicity and antiproliferative activity of the NT series
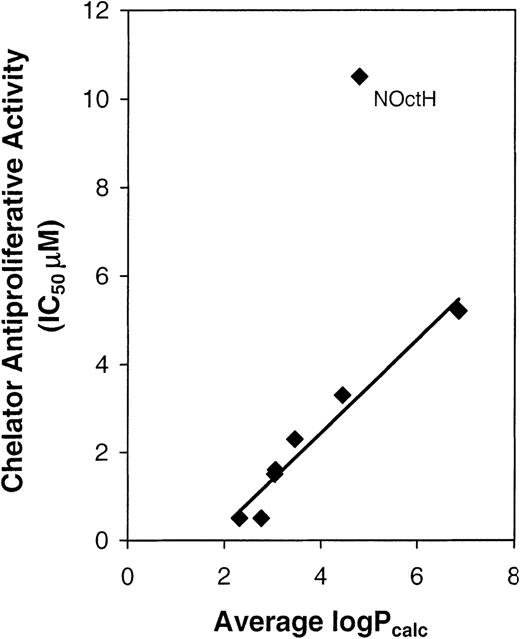
To assess the relationship between lipophilicity and antiproliferative activity, we plotted average calculated log P values (n-octanol-water partition coefficients) against IC50values in SK-N-MC cells (Figure 5). Log P values were calculated (Table 2) by the procedures of Broto et al,46 Ghose and Crippen,47 and Viswanadhan et al48 using Chem Draw Pro (v. 4.5, Cambridge Software, 1997). The means of these values (average calculated log P values; Table 2) showed a strong linear correlation with antiproliferative activity (r = 0.95; Figure 5) if NoctH was excluded. Possible reasons why NoctH did not fit the correlation may relate to structural differences relative to other analogues, namely, only NoctH has a highly lipophilic carbon “tail” (Figure 1B).
The relationship between the antiproliferative activity of the chelators using SK-N-MC neuroepithelioma cells (IC50values) and their lipophilicity (calculated log P values).
Log Pcalc values were estimated by the procedures of Broto et al,46 Ghose and Crippen,47 and Viswanadhan et al48 using the program Chem Draw (v. 4.5, 1997), and the results were then averaged (Table 2). Note that all average log Pcalc values were plotted (Table 2) except for chelators ST and N2mT, which had IC50 values greater than 12.5 μM and thus could not be graphed.
The relationship between the antiproliferative activity of the chelators using SK-N-MC neuroepithelioma cells (IC50values) and their lipophilicity (calculated log P values).
Log Pcalc values were estimated by the procedures of Broto et al,46 Ghose and Crippen,47 and Viswanadhan et al48 using the program Chem Draw (v. 4.5, 1997), and the results were then averaged (Table 2). Note that all average log Pcalc values were plotted (Table 2) except for chelators ST and N2mT, which had IC50 values greater than 12.5 μM and thus could not be graphed.
Our investigation identified a significant chelator structure–activity relationship dependent on lipophilicity. This was demonstrated by N4mT and N44mT, which have 1 and 2 methyl groups at N4 (Figure 1) and have IC50 values of 0.5 and 1.5 μM, respectively, in SK-N-MC cells. In addition, N4pT and N44pH (both more lipophilic than N4mT and N44mT; Table 2) have 1 and 2 phenyl groups at N4 and have IC50 values of 3.3 and 5.2 μM, respectively. Hence, for the NT series, antiproliferative activity against SK-N-MC cells decreased when the substituents on the terminal nitrogen atom of the thiosemicarbazide (N4; Figure 1A) became increasingly lipophilic.
The effect of the chelators on iron efflux from SK-N-MC neuroepithelioma cells
To determine the role of Fe chelation in the antiproliferative effects observed, we examined the ability of the ligands to mobilize59Fe from SK-N-MC cells. Cells were prelabeled for 3 hours at 37°C with 59Fe-Tf, washed, and then reincubated for 3 hours at 37°C with the chelators (Figure6A-B). As internal standards, DFO and 311 were used because their activities are well characterized.20,21,24 25
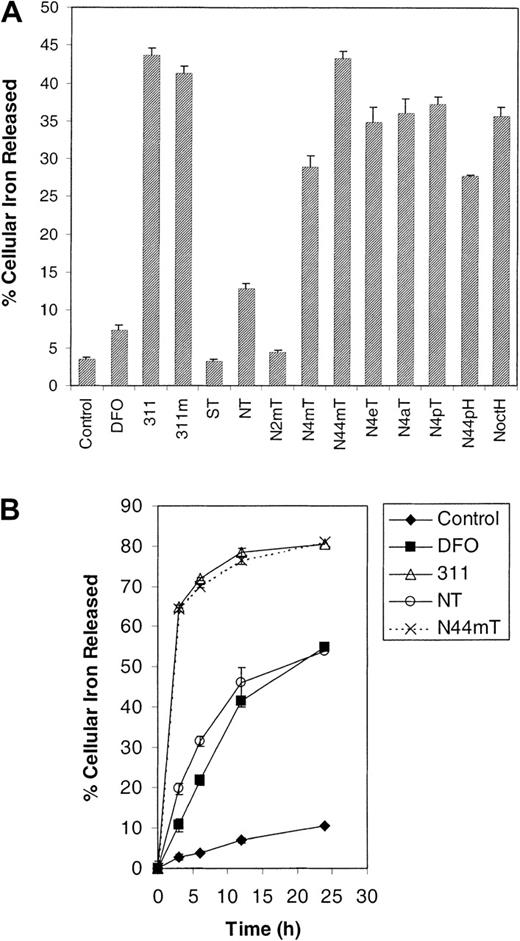
The effect of the chelators on 59Fe mobilization from prelabeled SK-N-MC neuroepithelioma cells.
Cells were labeled with 59Fe-Tf (0.75 μM) for 3 hours at 37°C, washed, and then (A) reincubated for 3 hours at 37°C in the presence of medium alone (control) or medium containing DFO (25 μM) or the other chelators (25 μM); or (B) reincubated for 3, 6, 12, and 24 hours. Results are expressed as the mean ± SD of 3 replicates in a typical experiment of 2 experiments performed.
The effect of the chelators on 59Fe mobilization from prelabeled SK-N-MC neuroepithelioma cells.
Cells were labeled with 59Fe-Tf (0.75 μM) for 3 hours at 37°C, washed, and then (A) reincubated for 3 hours at 37°C in the presence of medium alone (control) or medium containing DFO (25 μM) or the other chelators (25 μM); or (B) reincubated for 3, 6, 12, and 24 hours. Results are expressed as the mean ± SD of 3 replicates in a typical experiment of 2 experiments performed.
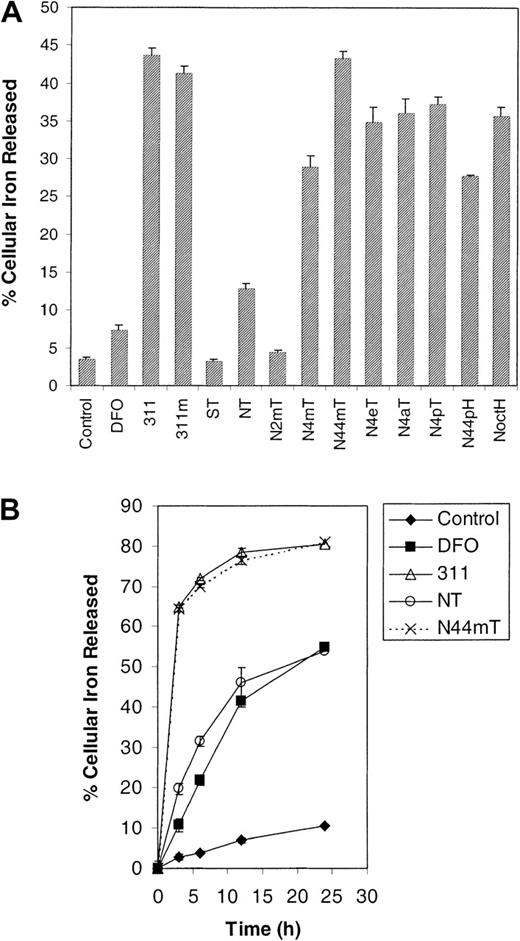
Broadly, the ligands can be grouped into 2 classes depending upon their ability to mobilize 59Fe. The first group has activity similar to DFO and includes ST, NT, and N2mT, which mobilize 3% to 13% of total cellular 59Fe (Figure 6A). The second group includes the remaining NT analogues, which are significantly (P < .0001) more effective than DFO, resulting in the release of 28% to 43% of cellular 59Fe (Figure 6A). Of this latter group, N44mT is the most efficient, having activity comparable to 311 and 311m and resulting in the release of 43% of59Fe (Figure 6A). The parent analogue, NT, mobilized only 13% of cellular 59Fe and was markedly less efficient than the other NT analogues (Figure 6A). The 59Fe mobilization observed for DFO and 311 correlated with that in our previous studies.21 24
Because NT has high antiproliferative efficacy (Figure 2; Table 1) but low activity at mobilizing 59Fe from cells (Figure 6A), time-course experiments were performed to understand its mechanism of action (Figure 6B). In these studies, N44mT was also assessed because it has high activity at inhibiting proliferation and mobilizing59Fe. These results with NT and N44mT were compared with those of 311 and DFO. Our experiments showed that DFO and NT behaved similarly, both increasing 59Fe efflux from the SK-N-MC cells as a function of time, being far less effective than N44mT and 311 (Figure 6B). These data suggest that NT and/or its Fe complex may be subject to similar permeability limitations as DFO, which forms a hydrophilic Fe complex that does not exit cells easily.49 50
The effect of the chelators on iron uptake from transferrin by SK-N-MC neuroepithelioma cells
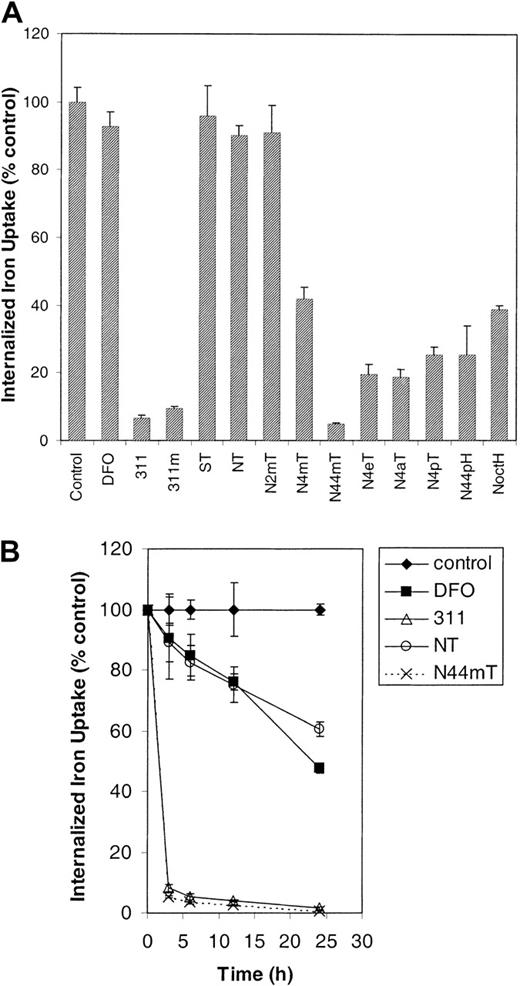
To further characterize the effects of the chelators on Fe metabolism, we examined the ability of the ligands to inhibit59Fe uptake from 59Fe-Tf (0.75 μM) by SK-N-MC cells in the absence or presence of the chelators (25 μM) for 3 hours at 37°C (Figure 7A-B). In agreement with their lower ability to mobilize 59Fe, ST, NT, and N2mT had little effect at preventing 59Fe uptake (Figure 7A). The most efficient chelators, namely 311, 311m, and N44mT, limited59Fe uptake to 5% to 9% of the control level, their activity being significantly greater (P < .0001) than that of DFO (Figure 7A). These results agree with the ability of these ligands to mobilize cellular 59Fe (Figure 6A). It is significant that all analogues (except ST, NT, and N2mT) showed much greater efficacy than DFO in preventing 59Fe uptake from59Fe-Tf by SK-N-MC cells (Figure 7A).
The effect of the chelators on 59Fe uptake from 59Fe-Tf by SK-N-MC neuroepithelioma cells.
(A) The cells were incubated for 3 hours at 37°C in medium containing59Fe-Tf (0.75 μM) and either DFO (25 μM) or the other chelators (25 μM), washed, and then incubated with pronase (1 mg/mL) for 30 minutes at 4°C to measure internalized 59Fe. (B) The cells were incubated as described in (A) for 3, 6, 12, and 24 hours at 37°C, and internalized 59Fe was measured using pronase. Results are expressed as the mean ± SD of 3 replicates in a typical experiment of 2 experiments performed.
The effect of the chelators on 59Fe uptake from 59Fe-Tf by SK-N-MC neuroepithelioma cells.
(A) The cells were incubated for 3 hours at 37°C in medium containing59Fe-Tf (0.75 μM) and either DFO (25 μM) or the other chelators (25 μM), washed, and then incubated with pronase (1 mg/mL) for 30 minutes at 4°C to measure internalized 59Fe. (B) The cells were incubated as described in (A) for 3, 6, 12, and 24 hours at 37°C, and internalized 59Fe was measured using pronase. Results are expressed as the mean ± SD of 3 replicates in a typical experiment of 2 experiments performed.
We examined whether the ability of the chelators to prevent59Fe uptake from 59Fe-Tf (Figure 7A) was due to direct removal of 59Fe from the protein. The most effective chelators at inhibiting proliferation, namely 311, 311m, NT, N4mT, and N44mT, were ineffective at removing 59Fe from Tf over the duration of an uptake experiment (3 hours), resulting in the release of 0.1% to 0.3% of the bound 59Fe, whereas DFO removed 0.7% (data not shown). These results demonstrate that the chelators have very little effect on direct 59Fe release from59Fe-Tf, as found for other aroylhydrazones.19Further, these data suggest that the ligands inhibit 59Fe uptake only after intracellular delivery from Tf.
Considering the limited permeability of NT and/or its Fe complex in kinetic studies assessing 59Fe mobilization (Figure 6B), we conducted time-course experiments examining the effects of NT on59Fe uptake from 59Fe-Tf. These results were compared with those of the most effective NT analogue, N44mT, and the standards 311 and DFO (Figure 7B). These experiments again showed that NT and/or its Fe complex may be subject to the same permeability limitations as DFO, as both chelators only moderately reduced59Fe uptake from 59Fe-Tf, even for incubations of 24 hours (Figure 7B). For example, after a 6-hour incubation, NT and DFO reduced 59Fe uptake by SK-N-MC cells to 82% and 85% of the control, respectively. In contrast, after 24 hours, NT and DFO reduced 59Fe uptake to 61% and 48% of the control, respectively (Figure 7B).
The relationship between iron-chelation efficacy and antiproliferative activity of the NT series in SK-N-MC neuroepithelioma cells
It was of interest to explore the relationship between Fe-chelation efficacy (Figures 6-7) and antiproliferative activity (Table 1; Figure 2). Chelators N4mT and N44mT have high chelation activity (Figures 6-7) and antiproliferative activity (IC50 = 0.5 and 1.5 μM, respectively; Table 1). However, N44pH and NoctH also demonstrate high Fe-chelation efficacy (Figures 6-7), but have much lower antiproliferative activity (IC50 = 5.2 and 10.5 μM, respectively; Table 1). Additionally, NT has marked antiproliferative effects (Table 1) but has low Fe-chelation efficacy (Figures 6-7). The lack of correlation between antiproliferative activity and Fe-chelation efficacy may reflect differences in the Fe pools targeted by these chelators and/or the relative efficacies of the ligand or its Fe complex to permeate membranes.
The effect of the chelators on [3H]thymidine incorporation by SK-N-MC neuroepithelioma cells
Because chelators can inhibit RR via Fe depletion,2the effect of the NT analogues on DNA synthesis in SK-N-MC cells was examined (Figure 8; Table3). Of the NT analogues, the most active inhibitor of DNA synthesis was N44mT (IC50 = 1.5 μM), which had significantly higher activity (P < .0001) than DFO (IC50 = 23 μM) and slightly less effect than 311 (IC50 = 0.6 μM; Figure 8; Table 3). N44mT also showed the highest Fe-chelation efficacy of the NT series in terms of increasing 59Fe mobilization (Figure 6A-B) and inhibiting 59Fe uptake (Figure 7A-B). On the other hand, ST, NT, and N2mT were among the least effective inhibitors of DNA synthesis (Figure 8; Table 3) and had relatively low Fe-chelation efficacy (Figures 6-7). However, overall, there was a very weak relationship between inhibiting DNA synthesis and either preventing 59Fe uptake from 59Fe-Tf (r = 0.48) or increasing 59Fe mobilization from prelabeled cells (r = 0.32). For example, in terms of inhibiting DNA synthesis, chelator NoctH was much less active (IC50 = 17.4 μM; Table 3) than N4pT (IC50 = 3.8 μM; Table 3), even though the ligands have comparable Fe-chelation efficacy (Figures 6-7). When the relationship between antiproliferative activity (Table 1) and [3H]thymidine incorporation (Table 3) was examined, a weak linear relationship was found (r = 0.54).
The effect of the chelators on [3H]thymidine incorporation by SK-N-MC neuroepithelioma cells.
Cells were seeded at 15 000 per well and allowed to grow overnight, and the chelators were then added in 0.1 mL of complete medium containing diferric transferrin (1.25 μM). After a 20-hour incubation at 37°C, [3H]thymidine (1 μCi [0.037 MBq]) was added and the cells were incubated for a further 2 hours at 37°C (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
The effect of the chelators on [3H]thymidine incorporation by SK-N-MC neuroepithelioma cells.
Cells were seeded at 15 000 per well and allowed to grow overnight, and the chelators were then added in 0.1 mL of complete medium containing diferric transferrin (1.25 μM). After a 20-hour incubation at 37°C, [3H]thymidine (1 μCi [0.037 MBq]) was added and the cells were incubated for a further 2 hours at 37°C (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.
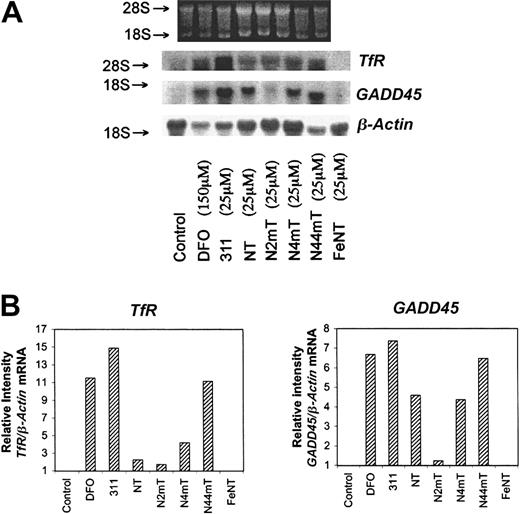
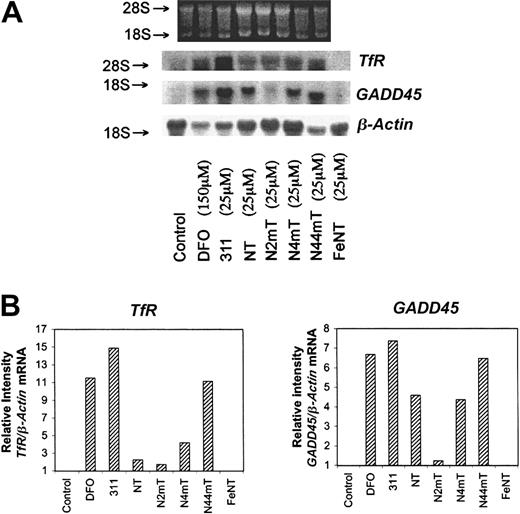
The effect of the chelators on the expression of TfR and cell-cycle control molecules
Low concentrations of 311 (25 μM) and much higher concentrations of DFO (150 μM) cause SK-N-MC cells to up-regulateTfR and GADD45 mRNA levels.25,27 The TfR plays a crucial role in Fe uptake,1,2 whereas GADD45 plays a role in cell-cycle arrest. Hence, it was of interest to assess whether the most active NT analogues also up-regulate these genes. In line with earlier studies,25 27 DFO (150 μM) and 311 (25 μM) up-regulated TfR and GADD45mRNA levels (Figure 9). Of the NT series, N44mT (25 μM) markedly up-regulated TfR andGADD45 mRNA levels, whereas NT, N4mT, and particularly N2mT showed less activity. The NT–Fe complex did not increaseTfR or GADD45 mRNA levels (Figure 9).
The effect of the chelators on the mRNA levels ofTfR, GADD45, and β-actin (loading control) in SK-N-MC neuroepithelioma cells.
Total RNA was extracted from cells after a 20-hour incubation with medium alone (control) or medium containing DFO (150 μM) or the other chelators (25 μM). The isolated RNA was subjected to electrophoresis on a 1.2% agarose-formaldehyde gel, transferred to a hybridization membrane, and probed under high-stringency conditions (see “Materials and methods”). The result illustrated is a typical experiment from 3 experiments performed.
The effect of the chelators on the mRNA levels ofTfR, GADD45, and β-actin (loading control) in SK-N-MC neuroepithelioma cells.
Total RNA was extracted from cells after a 20-hour incubation with medium alone (control) or medium containing DFO (150 μM) or the other chelators (25 μM). The isolated RNA was subjected to electrophoresis on a 1.2% agarose-formaldehyde gel, transferred to a hybridization membrane, and probed under high-stringency conditions (see “Materials and methods”). The result illustrated is a typical experiment from 3 experiments performed.
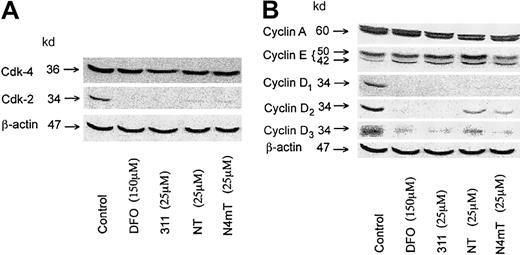
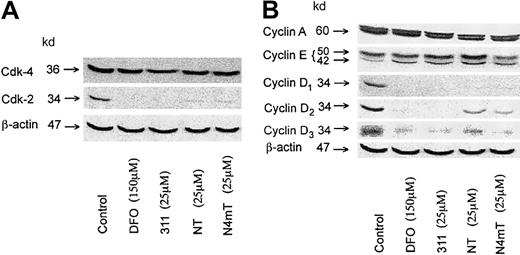
Because Fe deprivation causes G1/S arrest,2 we assessed changes in the expression of cell-cycle control molecules that play roles in G1/S progression. Hence, we examined the most cytotoxic NT analogues, namely NT and N4mT, compared with the known effects of 31127 on the protein levels of key cyclins (cyclins A, E, D1, D2, and D3) and cyclin-dependent kinases (cdk2 and cdk4). Hyperphosphorylation of the retinoblastoma gene susceptibility product (pRb) is essential for G1/S progression, and it is known that D-type cyclins bind cdk4 and/or cdk2 to phosphorylate pRb.51-54 In line with previous studies,27DFO (150 μM) and 311 (25 μM) caused a marked decrease in the expression of cyclins D1, D2, and D3 and also cdk2 in SK-N-MC cells (Figure 10A-B). Both NT (25 μM) and N4mT (25 μM) also caused a marked decrease in the expression of these cell-cycle control molecules, but were slightly less effective than 311 (Figure 10A-B). Relative to the control, all chelators had little effect on cdk4 and cyclin-A levels, whereas 311 and NT (25 μM) increased cyclin E (Figure 10A-B). These latter results may be due to cell-cycle dysregulation induced by the chelator.27
Effect of the NT analogues on the expression of cell-cycle control molecules.
The effect in SK-N-MC neuroepithelioma cells of the most cytotoxic NT analogues, NT and N4mT, compared with 311 on the protein levels of (A) cdk2, cdk4, and β-actin (loading control); or (B) cyclins A, E, D1, D2, D3, and β-actin (loading control). Western blot analysis was performed as described in “Materials and methods.” The results shown are typical of 2 experiments performed.
Effect of the NT analogues on the expression of cell-cycle control molecules.
The effect in SK-N-MC neuroepithelioma cells of the most cytotoxic NT analogues, NT and N4mT, compared with 311 on the protein levels of (A) cdk2, cdk4, and β-actin (loading control); or (B) cyclins A, E, D1, D2, D3, and β-actin (loading control). Western blot analysis was performed as described in “Materials and methods.” The results shown are typical of 2 experiments performed.
The effects of the chelators on iron-mediated free-radical damage
Doxorubicin and bleomycin partly mediate their antitumor effects by forming metal ion complexes that generate free radicals. We used the ascorbate oxidation, benzoate hydroxylation, and plasmid DNA cleavage assays to assess the redox activity of the Fe complexes of the chelators with the greatest antiproliferative activity in SK-N-MC cells, namely 311m, NT, and N4mT. The redox-active Fe complexes of EDTA and Triapine were used as controls because their effects are well characterized.40,55 56
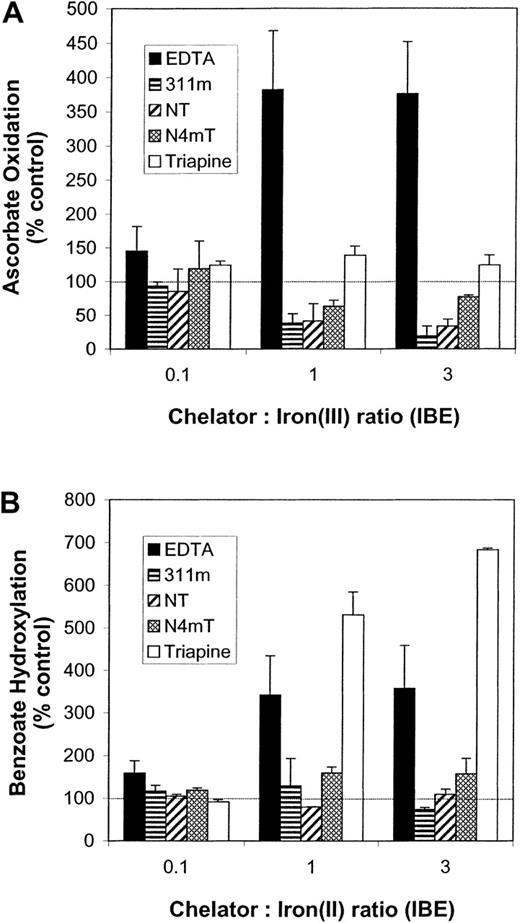
Ascorbate oxidation.
The ability of the Fe complexes of the ligands to reduce Fe(III) as determined by ascorbate oxidation is shown in Figure11A. EDTA promoted oxidation of ascorbate at all ligand–Fe(III) ratios (expressed as IBEs; see “Materials and methods”). At IBE ratios of 0.1, 1, and 3, EDTA increased ascorbate oxidation to 145%, 382%, and 376% of the control, respectively (Figure 11A). In contrast, 311m, NT, and N4mT at IBE ratios of 1 and 3 were protective against ascorbate oxidation (Figure 11A). For instance, at an IBE ratio of 3, these chelators reduced ascorbate oxidation to 19%, 33%, and 77% of the control, respectively (Figure 11A). Triapine potentiated ascorbate oxidation at all IBE ratios (Figure 11A).
Effects of the chelators on iron-mediated oxygen radical production as judged by ascorbate oxidation and benzoate hydroxylation.
The effects of EDTA, 311m, Triapine, and the most cytotoxic NT analogues (NT and N4mT) on (A) the Fe(III)-induced oxidation of ascorbate and (B) the Fe(II)-induced hydroxylation of benzoate. Ascorbate oxidation and benzoate hydroxylation were assessed as described in “Materials and methods.” The results are the mean ± SEM of 3 experiments.
Effects of the chelators on iron-mediated oxygen radical production as judged by ascorbate oxidation and benzoate hydroxylation.
The effects of EDTA, 311m, Triapine, and the most cytotoxic NT analogues (NT and N4mT) on (A) the Fe(III)-induced oxidation of ascorbate and (B) the Fe(II)-induced hydroxylation of benzoate. Ascorbate oxidation and benzoate hydroxylation were assessed as described in “Materials and methods.” The results are the mean ± SEM of 3 experiments.
Benzoate hydroxylation.
This assay is based on the ability of hydroxyl radicals to hydroxylate benzoate to fluorescent products (308 nm excitation and 410 nm emission) (T. Chaston, D.B.L., R. N. Watts, D.R.R., manuscript submitted, Dean and Nicholson,43 and Gutteridge44). Previous studies showed that the EDTA–Fe complex increases benzoate hydroxylation.43 At IBE ratios of 0.1, 1, and 3, EDTA increased benzoate hydroxylation to 159%, 341%, and 358% of the control, respectively (Figure 11B). Chelator 311m was mildly protective at an IBE ratio of 3, at which it reduced benzoate hydroxylation to 73% of the control (Figure 11B). NT had little effect on benzoate hydroxylation, whereas N4mT elevated it to 157% of the control (Figure 11B). In contrast, Triapine greatly increased benzoate hydroxylation to 530% and 683% of the control at IBE ratios of 1 and 3, respectively (Figure 11B).
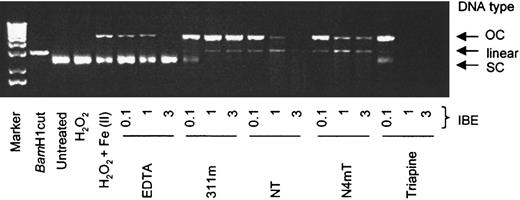
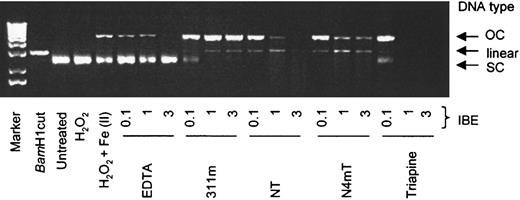
Plasmid DNA integrity.
In these studies, we examined the ability of chelators to prevent Fe-mediated hydroxyl-radical damage to plasmid DNA.45Untreated plasmid and plasmid treated with H2O2appeared on gels as a single supercoiled (SC) DNA band (Figure12). As another control, plasmid treated with BamHI was included, resulting in a single band of linearized DNA (Figure 12). When plasmid was treated with Fe(II) and H2O2, SC DNA was partially converted to the open circular (OC) form (Figure 12).
Redox activity of the Fe complexes of the chelators assessed by plasmid DNA cleavage assays.
The effects of EDTA, 311m, Triapine, and the most cytotoxic NT analogues (NT and N4mT) on integrity of the plasmid pGEM-7Zf(+) when incubated in the presence of Fe(II) and hydrogen peroxide. Reagents were added in the following order: purified sterile water, chelator (1, 10, and 30 μM), FeSO4 (10 μM), H2O2 (1 mM), and plasmid (10 μg/mL); followed by incubation at room temperature for 30 minutes before loading onto a 1% agarose gel (see “Materials and methods”). Results are a typical experiment from 8 performed.
Redox activity of the Fe complexes of the chelators assessed by plasmid DNA cleavage assays.
The effects of EDTA, 311m, Triapine, and the most cytotoxic NT analogues (NT and N4mT) on integrity of the plasmid pGEM-7Zf(+) when incubated in the presence of Fe(II) and hydrogen peroxide. Reagents were added in the following order: purified sterile water, chelator (1, 10, and 30 μM), FeSO4 (10 μM), H2O2 (1 mM), and plasmid (10 μg/mL); followed by incubation at room temperature for 30 minutes before loading onto a 1% agarose gel (see “Materials and methods”). Results are a typical experiment from 8 performed.
Although EDTA was redox active in the ascorbate oxidation (Figure 11A) and benzoate hydroxylation assays (Figure 11B), this chelator was protective of SC DNA at IBE ratios of 1 and 3. This agrees with the well-characterized protective effects of EDTA against Fe-mediated DNA damage. However, 311m was not protective of SC DNA in the presence of Fe(II) and H2O2, resulting in the formation of OC DNA and also linearized DNA, particularly at IBE ratios of 1 and 3 (Figure 12). These findings were observed even though 311m was redox inactive in the ascorbate oxidation and benzoate hydroxylation assays (Figure 11A-B). The reason for this inconsistency may be that the PIH class of chelators has low affinity for Fe(II).57Additionally, differences in the interactions of 311m and/or Fe(II) with benzoate and DNA may contribute to this discrepancy.
The Fe complexes of NT and N4mT at an IBE of 0.1 showed effects on SC DNA that were similar to those of the Fe complex of 311m (Figure 12). However, at IBE ratios of 1 and 3, N4mT and particularly NT caused plasmid degradation. This latter effect was pronounced for NT at an IBE of 3 (Figure 12). Triapine was more aggressive than NT, completely degrading plasmid DNA at IBE ratios of 1 and 3 (Figure 12).
Discussion
New strategies for selectively inhibiting cancer cell growth are vital, especially considering that some tumors become resistant to conventional chemotherapy. Considering this, many studies using cell culture, animal models, and clinical trials have shown that tumors are sensitive to Fe-chelation therapy using DFO.2,11-17However, DFO has serious disadvantages, including its requirement for long infusions, its short plasma half-life, and its poor permeability, which leads to poor antitumor activity.58 59 Hence, it is essential to examine new chelators with greater activity.
We have designed novel “hybrid” Fe chelators known as the NT series. These ligands incorporate structural elements of the highly active thiosemicarbazones28-31 with the features of the most effective PIH analogues.21 24 Our current studies have identified new ligands that show selective antitumor activity that is much greater than that of DFO. These results confirm our design strategy and provide insights into the structural requirements for optimal antitumor activity. In contrast to previous chelators of the PIH class, our new ligands are the subject of a patent application. This is important in terms of characterizing these agents as antitumor drugs because pharmaceutical development will not proceed without patent protection.
One problem with all cytotoxic agents for cancer treatment is the lack of selectivity against tumor cells as opposed to normal cells. The use of Fe as a target is a relatively new therapeutic strategy that has shown promising results, even using DFO, which is not highly membrane permeable.11-17 More permeable lipophilic chelators that rapidly inhibit RR show more promise. Indeed, Triapine is already in phase II clinical trials and demonstrates high activity and appreciable selectivity.30,31 Some of our ligands are more effective chelators than Triapine,41 and we have shown that 311 is a potent RR inhibitor that can overcome hydroxyurea resistance in vitro.60
Significantly, the most cytotoxic NT analogues against SK-N-MC neuroepithelioma cells, namely NT and N4mT, showed selective antitumor activity, having significantly less effect on a range of normal cells (Table 1). However, although these compounds showed high activity against neuroepithelioma cells, less efficacy was observed in other tumor cell types (Table 1). These results indicate that NT and N4mT may have more potential in the management of neuroepithelioma and related tumors. Although N44mT showed slightly less antiproliferative activity against SK-N-MC cells than NT or N4mT, this ligand displayed high efficacy against K562 leukemia and SK-Mel-28 melanoma cells. In addition to these “hybrid” ligands, we also identified the 311 analogue 311m, which shows high antitumor activity against neoplastic cells and significantly less activity against normal cells (Table 1).
The basis of selectivity for the chelators is that rapidly growing tumor cells have a higher Fe requirement for DNA synthesis and metabolism than more slowly growing normal cells. Indeed, the more slowly growing fibroblasts (doubling time, 22 hours) were far less affected than the more rapidly growing SK-N-MC neuroepithelioma cells (doubling time, 16 hours) (Table 1). Considering the effect on normal cells, it was significant that one of the most potent Fe chelators studied, 311m, showed similar activity to cisplatin and far less effect than doxorubicin at inhibiting the growth of bone marrow stem cells. Furthermore, injection of 311 intraperitoneally (300 mg · kg−1 · d−1; 5 d/wk) in nude mice bearing an NB xenograft resulted in no marked deleterious effects over 4 weeks, but resulted in a 60% decrease (n = 4) in tumor size (data not shown). These results, together with studies showing the activity of aroylhydrazones against tumors in vivo61,62 in the absence of pronounced toxicity,62 demonstrate their potential as anticancer agents.
Considering the role of Fe in proliferation1,2 and the fact that tumors are sensitive to Fe depletion,2 it was vital to determine the Fe-chelation efficacy of the ligands. Our studies showed that the Fe-chelation efficacy of most NT ligands was higher than that of DFO. However, NT demonstrated Fe-chelation efficacy comparable to DFO. In light of its high antiproliferative activity, it is possible that the lower chelation efficacy of NT may be due to the formation of an Fe complex that is less lipophilic and subject to impaired permeability. It is relevant to discuss that chelator N2mT showed no appreciable Fe-chelation activity. These data are in agreement with its low antiproliferative efficacy (Table 1) and reflect its difficulty in coordinating to Fe. In this case, the methyl group at the 2-position of N2mT (Figure 1) hinders the electron delocalization that stabilizes Fe binding. Nonetheless, this result, together with the fact that the NT–Fe complex shows far less antiproliferative activity than NT and no ability to induce TfR or GADD45mRNA expression (Figure 9), suggests that the antiproliferative activity of the NT series is due to their ability to bind Fe.
In the present study, NT was one of the most effective ligands in terms of its ability to inhibit proliferation, despite its lower Fe-chelation efficacy than other NT analogues or 311. The fact that NT does not markedly deplete cells of Fe could be an advantage over 311 and 311m, which are the most effective chelators ever assessed in our laboratories. One possible problem with 311 and 311m is their potential to markedly deplete Fe levels within the organism. However, the use of NT, which does not possess such high Fe-chelation efficacy, could be more appropriate. Obviously, detailed dose-response studies in animals should be conducted, and these are under way. Indeed, the high antiproliferative activity of these chelators (Table 1) and marked Fe-chelation efficacy (eg, 311m; Figures 6-7) could necessitate very low doses, preventing profound Fe depletion but maintaining antitumor activity.
Because thiosemicarbazones are RR inhibitors,63,64 we assessed the effects of our chelators on DNA synthesis. For 311, 311m, and N44mT, inhibition of DNA synthesis (Table 3) was associated with high Fe-chelation efficacy (Figures 6A,7A) and antiproliferative activity (Table 1). However, for some chelators there was less correlation. For instance, although NT markedly inhibited proliferation (Table 1), it only moderately prevented DNA synthesis (Table 3), perhaps reflecting its lower Fe-chelation efficacy. The modest prevention of DNA synthesis by NT indicates that it has other modes of action besides inhibition of RR. Indeed, we showed that these chelators also affect the expression of cell-cycle control molecules (Figure 10). It should also be noted that our previous studies20 21showed that there was a lack of correlation between Fe-chelation efficacy and antiproliferative activity. This probably reflects the different cellular Fe pools (which have divergent functions) that are targeted by the chelators.
Our studies of the aroylhydrazone chelators with the same Fe-binding site as 311, namely 311m, N44pH, and NoctH, demonstrate that these ligands show high affinity and selectivity for Fe(III) and much lower affinity for Mg(II), Ca(II), and Zn(II).57,65 Further, thiosemicarbazones are well-known chelators with high avidity for Fe.66-68 Formation-constant calculations show that all our chelators (apart from N2mT) have similar and high Fe-binding affinity and selectivity for this metal (D.R.R., in preparation). In addition, there was no defined correlation between Fe-binding affinity and biologic activity. Indeed, our previous studies showed that for aroylhydrazone ligands, one of the most important effects on activity appears to be lipophilicity.19 Considering the affinity and selectivity of one of our most active chelators, 311m, at pH 7.4 using a ligand concentration of 10−3 M and a metal concentration of 10−6 M, the −log of the free metal concentration (pM) for Fe(III) was 28. In contrast, the affinity of this ligand for Ca(II) and Mg(II) was negligible (pM = 6). As observed for DFO and PIH,57 the chelator had a higher affinity for Zn(II), with a pM of 10. Hence, the selectivity of our aroylhydrazones for Fe was similar to that of DFO and PIH.57
Another mode of activity exerted by NT analogues may be the formation of Fe complexes that generate toxic free radicals. Thiosemicarbazone–Fe complexes have antitumor activity and inhibit RR,63,64 and in fact, Triapine30 forms an Fe complex with marked antiproliferative and redox activity (Figure 11; T. Chaston, D.B.L., R. N. Watts, D.R.R., manuscript submitted). In contrast, the NT–Fe complex was relatively inactive in terms of antitumor activity (Table 1), and both the Fe complexes of NT and N4mT failed to oxidize ascorbate or hydroxylate benzoate (Figure 11). However, NT did degrade DNA at an IBE ratio of 3, although its effect was far less than that of Triapine (Figure 12). Thus, the redox activity of NT and N4mT was less than that of Triapine and may play a lesser role in their biologic effects.
In summary, the combination of 2-hydroxy-1-naphthylaldehyde with various thiosemicarbazides results in novel hybrid chelators with significantly greater antiproliferative activity than DFO. We demonstrated that this activity was selective for tumor cells, as the most efficient analogues were less effective at inhibiting the growth of normal cells. Because of these properties, NT, N4mT, and N44mT, as well as the 311 analogue 311m, warrant further investigation for cancer chemotherapy.
We thank Ms Juliana Kwok for her suggestions concerning the manuscript prior to submission. We gratefully acknowledge Mr Tim Chaston for his expertise in assisting with redox activity studies and Ms Vicky Antonenas, Department of Hematology, Institute of Clinical Pathology and Medical Research, Sydney, for help with bone marrow stem cell studies.
Supported by grants from the National Health and Medical Research Council and an Australian Research Council Large Grant. The Heart Research Institute also provided financial support.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Des R. Richardson, The Heart Research Institute, 145 Missenden Rd, Camperdown, Sydney, New South Wales, 2050 Australia; e-mail: d.richardson@hri.org.au.



![Fig. 8. The effect of the chelators on [3H]thymidine incorporation by SK-N-MC neuroepithelioma cells. / Cells were seeded at 15 000 per well and allowed to grow overnight, and the chelators were then added in 0.1 mL of complete medium containing diferric transferrin (1.25 μM). After a 20-hour incubation at 37°C, [3H]thymidine (1 μCi [0.037 MBq]) was added and the cells were incubated for a further 2 hours at 37°C (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood.v100.2.666/6/m_h81422839008.jpeg?Expires=1768065225&Signature=kB-QG2sVyVdGl0w14SyX1eG3hqnXPpl03v9D2ArDCRM4yBbEn~NtHdCnSUZqNtXBkK4A45HD5lgvnEx9hry74Gi6BFGnJ374CoxgU8V3-KOWhXLcD05hMV-TtygraOQOYHS9-xtFXl3XJKRaiSxQBvIFSXQH4dyVo983g4JkqrKdQ6GbnOgI--E23jJuEGRRZiubCqEw3cNIc7oacNas~JIgFZlSO8BKLA21-43cG1XqpZ0IF-tHp5DLy5FlTrKQmj11elU1ZF0nEs6nyBVm8bnbqaOSJrODTl-IB8D4K5UvBXkgAVCVm0rCAOqvpQVwWBaRSe1x0X7sBmCtIpltVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Fig. 8. The effect of the chelators on [3H]thymidine incorporation by SK-N-MC neuroepithelioma cells. / Cells were seeded at 15 000 per well and allowed to grow overnight, and the chelators were then added in 0.1 mL of complete medium containing diferric transferrin (1.25 μM). After a 20-hour incubation at 37°C, [3H]thymidine (1 μCi [0.037 MBq]) was added and the cells were incubated for a further 2 hours at 37°C (see “Materials and methods”). Each data point represents the mean of 2 replicates in a typical experiment of 3 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood.v100.2.666/6/m_h81422839008.jpeg?Expires=1768238293&Signature=SQPfd3qsbNQeJCAftn8Inzxqs1JAkNmac9zq~4dtW~rCwQlfaxV3BpK-TLZEVxBewJHq-HR4G5nshuWA9pyALl5-eMW8RJHBy-z3Lffih7kcmWvTufFOgPbbrCBuBPmOzdAWWl4ffpyMXUt7fOZHijc-q1-vmvYH0HsiY2mhKAAbDBSg7YFo1PKJ1IQEsufpUbdzZPiwWUtsIze1K4HxHflnWO0NEAQ~kyzoVOamQpTbhyXLlFLfyf3E5IN8ZUTnPSMiJwL0EgA8C5swAYgNpDVi~JDbA3CJYLPiAXk0bWuiVJiVAd9Fs7ij4Mp6poXrw66GERGv6yG~ih8D6JRlvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)